A Cell Line for Detection of Botulinum Neurotoxin Type B
- PMID: 29170639
- PMCID: PMC5684488
- DOI: 10.3389/fphar.2017.00796
A Cell Line for Detection of Botulinum Neurotoxin Type B
Abstract
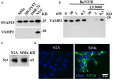
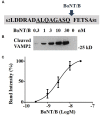
Botulinum neurotoxins (BoNTs) type A and type B are commonly used as biopharmaceutics for neurological diseases, uniquely allowing months-long paralysis of target muscles. Their exquisite neuronal specificity is conferred by a multistep process of binding, internalization, cytosolic escape and cleavage of the neuron-specific proteins, SNAP-25 and vesicle-associated membrane proteins (VAMPs), ultimately to inhibit secretion of neurotransmitters. Currently the mouse lethality bioassay is the only available method for quality control testing of VAMP-cleaving botulinum products. Refined assays for botulinum product testing are urgently needed. Specifically, in vitro replacement assays which can account for all steps of BoNT intoxication are in high demand. Here, we describe a novel SiMa cell-based approach where re-engineering of the VAMP molecule allows detection of all BoNT/B intoxication steps using a luminescent enzymatic reaction with sensitivity comparable to mouse LD50 bioassay. The presented one-step enzyme-linked immunosorbent assay meets 3Rs (replacement, reduction, and refinement of the use of animals) objectives, is user-friendly and will accelerate development of new botulinum drugs. The sensitive enzymatic reporter cell line could also be adapted for the detection of toxin activity during the manufacture of botulinum and tetanus vaccines.
Keywords: 3Rs; Myobloc; SiMa neuroblastoma; VAMP; botulinum neurotoxin type B; botulinum neurotoxin-sensitive cell line; luciferase; tetanus.
Figures


Comment in
-
Commentary: A Cell Line for Detection of Botulinum Neurotoxin Type B.Front Pharmacol. 2018 Sep 25;9:1056. doi: 10.3389/fphar.2018.01056. eCollection 2018. Front Pharmacol. 2018. PMID: 30319408 Free PMC article. No abstract available.
References
-
- Adler S., Bicker G., Bigalke H., Bishop C., Blümel J., Dressler D., et al. (2010). The current scientific and legal status of alternative methods to the LD50 test for Botulinum neurotoxin potency testing. ATLA 38 315–330. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

