Shedding of Microvesicles from Microglia Contributes to the Effects Induced by Metabotropic Glutamate Receptor 5 Activation on Neuronal Death
- PMID: 29170640
- PMCID: PMC5684115
- DOI: 10.3389/fphar.2017.00812
Shedding of Microvesicles from Microglia Contributes to the Effects Induced by Metabotropic Glutamate Receptor 5 Activation on Neuronal Death
Abstract
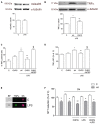
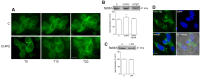
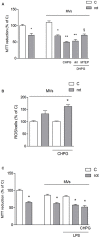
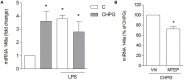
Metabotropic glutamate (mGlu) receptor 5 is involved in neuroinflammation and has been shown to mediate reduced inflammation and neurotoxicity and to modify microglia polarization. On the other hand, blockade of mGlu5 receptor results in inhibition of microglia activation. To dissect this controversy, we investigated whether microvesicles (MVs) released from microglia BV2 cells could contribute to the communication between microglia and neurons and whether this interaction was modulated by mGlu5 receptor. Activation of purinergic ionotropic P2X7 receptor with the stable ATP analog benzoyl-ATP (100 μM) caused rapid MVs shedding from BV2 cells. Ionic currents through P2X7 receptor increased in BV2 cells pretreated for 24 h with the mGlu5 receptor agonist CHPG (200 μM) as by patch-clamp recording. This increase was blunted when microglia cells were activated by exposure to lipopolysaccharide (LPS; 0.1 μg/ml for 6 h). Accordingly, a greater amount of MVs formed after CHPG treatment, an effect prevented by the mGlu5 receptor antagonist MTEP (100 μM), as measured by expression of flotillin, a membrane protein enriched in MVs. Transferred MVs were internalized by SH-SY5Y neurons where they did not modify neuronal death induced by a low concentration of rotenone (0.1 μM for 24 h), but significantly increased rotenone neurotoxicity when shed from CHPG-treated BV2 cells. miR146a was increased in CHPG-treated MVs, an effect concealed in MVs from LPS-activated BV2 cells that showed per se an increase in miRNA146a levels. The present data support a role for microglia-shed MVs in mGlu5-mediated modulation of neuronal death and identify miRNAs as potential critical mediators of this interaction.
Keywords: CHPG; extracellular vesicle; mGlu5; miRNA; microglia; neuroinflammation.
Figures

References
-
- Aronica E., van Vliet E. A., Mayboroda O. A., Troost D., da Silva F. H., Gorter J. A. (2000). Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur. J. Neurosci. 12 2333–2344. 10.1046/j.1460-9568.2000.00131.x - DOI - PubMed
-
- Berthele A., Boxall S. J., Urban A., Anneser J. M., Zieglgansberger W., Urban L., et al. (1999). Distribution and developmental changes in metabotropic glutamate receptor messenger RNA expression in the rat lumbar spinal cord. Brain Res. Dev. Brain Res. 112 39–53. 10.1016/S0165-3806(98)00156-4 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

