Bioresorbable Vascular Scaffold Korean Expert Panel Report
- PMID: 29171214
- PMCID: PMC5711671
- DOI: 10.4070/kcj.2017.0300
Bioresorbable Vascular Scaffold Korean Expert Panel Report
Abstract
Bioresorbable vascular scaffold (BRS) is an innovative device that provides structural support and drug release to prevent early recoil or restenosis, and then degrades into nontoxic compounds to avoid late complications related with metallic drug-eluting stents (DESs). BRS has several putative advantages. However, recent randomized trials and registry studies raised clinical concerns about the safety and efficacy of first generation BRS. In addition, the general guidance for the optimal practice with BRS has not been suggested due to limited long-term clinical data in Korea. To address the safety and efficacy of BRS, we reviewed the clinical evidence of BRS implantation, and suggested the appropriate criteria for patient and lesion selection, scaffold implantation technique, and management.
Keywords: Bioresorbable vascular scaffold; Coronary disease; Stents; Thrombosis.
Copyright © 2017. The Korean Society of Cardiology.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

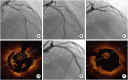
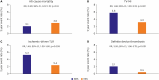



References
-
- Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015;385:43–54. - PubMed
-
- Karanasos A, Simsek C, Gnanadesigan M, et al. OCT assessment of the long-term vascular healing response 5 years after everolimus-eluting bioresorbable vascular scaffold. J Am Coll Cardiol. 2014;64:2343–2356. - PubMed
-
- Brugaletta S, Radu MD, Garcia-Garcia HM, et al. Circumferential evaluation of the neointima by optical coherence tomography after ABSORB bioresorbable vascular scaffold implantation: can the scaffold cap the plaque? Atherosclerosis. 2012;221:106–112. - PubMed
-
- Simsek C, Karanasos A, Magro M, et al. Long-term invasive follow-up of the everolimus-eluting bioresorbable vascular scaffold: five-year results of multiple invasive imaging modalities. EuroIntervention. 2016;11:996–1003. - PubMed
-
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016;388:2479–2491. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

