Locomotor analysis identifies early compensatory changes during disease progression and subgroup classification in a mouse model of amyotrophic lateral sclerosis
- PMID: 29171432
- PMCID: PMC5696848
- DOI: 10.4103/1673-5374.217346
Locomotor analysis identifies early compensatory changes during disease progression and subgroup classification in a mouse model of amyotrophic lateral sclerosis
Abstract
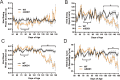
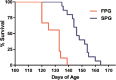
Amyotrophic lateral sclerosis is a motoneuron degenerative disease that is challenging to diagnose and presents with considerable variability in survival. Early identification and enhanced understanding of symptomatic patterns could aid in diagnosis and provide an avenue for monitoring disease progression. Use of the mSOD1G93A mouse model provides control of the confounding environmental factors and genetic heterogeneity seen in amyotrophic lateral sclerosis patients, while investigating underlying disease-induced changes. In the present study, we performed a longitudinal behavioral assessment paradigm and identified an early hindlimb symptom, resembling the common gait abnormality foot drop, along with an accompanying forelimb compensatory mechanism in the mSOD1G93A mouse. Following these initial changes, mSOD1 mice displayed a temporary hindlimb compensatory mechanism resembling an exaggerated steppage gait. As the disease progressed, these compensatory mechanisms were not sufficient to sustain fundamental locomotor parameters and more severe deficits appeared. We next applied these initial findings to investigate the inherent variability in B6SJL mSOD1G93A survival. We identified four behavioral variables that, when combined in a cluster analysis, identified two subpopulations with different disease progression rates: a fast progression group and a slow progression group. This behavioral assessment paradigm, with its analytical approaches, provides a method for monitoring disease progression and detecting mSOD1 subgroups with different disease severities. This affords researchers an opportunity to search for genetic modifiers or other factors that likely enhance or slow disease progression. Such factors are possible therapeutic targets with the potential to slow disease progression and provide insight into the underlying pathology and disease mechanisms.
Keywords: SOD1 mouse; amyotrophic lateral sclerosis; disease progression; disease variability; locomotor; motoneuron degenerative disease; nerve regeneration; neural regeneration.
Conflict of interest statement
We have no conflict of interests
Figures





Similar articles
-
Identification of B6SJL mSOD1(G93A) mouse subgroups with different disease progression rates.J Comp Neurol. 2015 Dec 15;523(18):2752-68. doi: 10.1002/cne.23814. Epub 2015 Jun 22. J Comp Neurol. 2015. PMID: 26010802 Free PMC article.
-
Transcriptomic indices of fast and slow disease progression in two mouse models of amyotrophic lateral sclerosis.Brain. 2013 Nov;136(Pt 11):3305-32. doi: 10.1093/brain/awt250. Epub 2013 Sep 24. Brain. 2013. PMID: 24065725
-
Axonal degeneration, distal collateral branching and neuromuscular junction architecture alterations occur prior to symptom onset in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis.J Chem Neuroanat. 2016 Oct;76(Pt A):35-47. doi: 10.1016/j.jchemneu.2016.03.003. Epub 2016 Mar 30. J Chem Neuroanat. 2016. PMID: 27038603
-
CD4 + T Cells and Neuroprotection: Relevance to Motoneuron Injury and Disease.J Neuroimmune Pharmacol. 2015 Dec;10(4):587-94. doi: 10.1007/s11481-015-9625-x. Epub 2015 Jul 7. J Neuroimmune Pharmacol. 2015. PMID: 26148561 Free PMC article. Review.
-
Transgenic mice with human mutant genes causing Parkinson's disease and amyotrophic lateral sclerosis provide common insight into mechanisms of motor neuron selective vulnerability to degeneration.Rev Neurosci. 2007;18(2):115-36. doi: 10.1515/revneuro.2007.18.2.115. Rev Neurosci. 2007. PMID: 17593875 Review.
Cited by
-
Delineating sex-dependent and anatomic decline of motor functions in the SOD1G93A mouse model of amyotrophic lateral sclerosis.bioRxiv [Preprint]. 2024 Dec 17:2024.12.17.628968. doi: 10.1101/2024.12.17.628968. bioRxiv. 2024. PMID: 39763885 Free PMC article. Preprint.
-
Automated gait analysis indicates efficacy of T-type calcium channel inhibition for mitigation of disrupted calcium signalling in an SCA5 mouse model.Sci Rep. 2025 Jul 1;15(1):20990. doi: 10.1038/s41598-025-05511-1. Sci Rep. 2025. PMID: 40594196 Free PMC article.
-
Metformin and tBHQ Treatment Combined with an Exercise Regime Prevents Osteosarcopenic Obesity in Middle-Aged Wistar Female Rats.Oxid Med Cell Longev. 2021 Aug 14;2021:5294266. doi: 10.1155/2021/5294266. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34447486 Free PMC article.
-
A novel assessment of fine-motor function reveals early hindlimb and detectable forelimb deficits in an experimental model of ALS.Sci Rep. 2022 Oct 11;12(1):17010. doi: 10.1038/s41598-022-20333-1. Sci Rep. 2022. PMID: 36220871 Free PMC article.
-
Carboxyl-terminal modulator protein regulates Akt signaling during skeletal muscle atrophy in vitro and a mouse model of amyotrophic lateral sclerosis.Sci Rep. 2019 Mar 8;9(1):3920. doi: 10.1038/s41598-019-40553-2. Sci Rep. 2019. PMID: 30850672 Free PMC article.
References
-
- Abe K, Aoki M, Ikeda M, Watanabe M, Hirai S, Itoyama Y. Clinical characteristics of familial amyotrophic lateral sclerosis with Cu/Zn superoxide dismutase gene mutations. J Neurol Sci. 1996;136:108–116. - PubMed
-
- Alexander NB, Goldberg A. Gait disorders: search for multiple causes. Cleve Clin J Med. 2005;72(586):589–590. 592-584 passim. - PubMed
-
- Azzouz M, Leclerc N, Gurney M, Warter JM, Poindron P, Borg J. Progressive motor neuron impairment in an animal model of familial amyotrophic lateral sclerosis. Muscle Nerve. 1997;20:45–51. - PubMed
-
- Ben Hamida M, Hentati F, Ben Hamida C. Hereditary motor system diseases (chronic juvenile amyotrophic lateral sclerosis), Conditions combining a bilateral pyramidal syndrome with limb and bulbar amyotrophy. Brain. 1990;113(Pt 2):347–363. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

