EPO-releasing neural precursor cells promote axonal regeneration and recovery of function in spinal cord traumatic injury
- PMID: 29172009
- PMCID: PMC5701768
- DOI: 10.3233/RNN-170750
EPO-releasing neural precursor cells promote axonal regeneration and recovery of function in spinal cord traumatic injury
Abstract
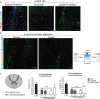
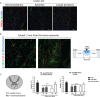
Background: Spinal cord injury (SCI) is a debilitating condition characterized by a complex of neurological dysfunctions ranging from loss of sensation to partial or complete limb paralysis. Recently, we reported that intravenous administration of neural precursors physiologically releasing erythropoietin (namely Er-NPCs) enhances functional recovery in animals following contusive spinal cord injury through the counteraction of secondary degeneration. Er-NPCs reached and accumulated at the lesion edges, where they survived throughout the prolonged period of observation and differentiated mostly into cholinergic neuron-like cells.
Objective: The aim of this study was to investigate the potential reparative and regenerative properties of Er-NPCs in a mouse experimental model of traumatic spinal cord injury.
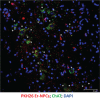
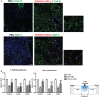
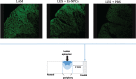
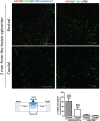
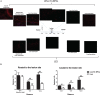
Methods and results: We report that Er-NPCs favoured the preservation of axonal myelin and strongly promoted the regrowth across the lesion site of monoaminergic and chatecolaminergic fibers that reached the distal portions of the injured cord. The use of an anterograde tracer transported by the regenerating axons allowed us to assess the extent of such a process. We show that axonal fluoro-ruby labelling was practically absent in saline-treated mice, while it resulted very significant in Er-NPCs transplanted animals.
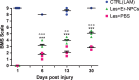
Conclusion: Our study shows that Er-NPCs promoted recovery of function after spinal cord injury, and that this is accompanied by preservation of myelination and strong re-innervation of the distal cord. Thus, regenerated axons may have contributed to the enhanced recovery of function after SCI.
Keywords: Spinal cord injury; animal behavior; neural stem cells; regenerative medicine; transplantation.
Figures

Similar articles
-
Exogenous adult postmortem neural precursors attenuate secondary degeneration and promote myelin sparing and functional recovery following experimental spinal cord injury.Cell Transplant. 2015;24(4):703-19. doi: 10.3727/096368914X685140. Epub 2014 Oct 8. Cell Transplant. 2015. PMID: 25299753
-
Grafted Neural Precursors Integrate Into Mouse Striatum, Differentiate and Promote Recovery of Function Through Release of Erythropoietin in MPTP-Treated Mice.ASN Neuro. 2016 Oct 27;8(5):1759091416676147. doi: 10.1177/1759091416676147. Print 2016 Oct. ASN Neuro. 2016. PMID: 27789613 Free PMC article.
-
Transplanted bone marrow stromal cells promote axonal regeneration and improve motor function in a rat spinal cord injury model.Neurosurgery. 2009 May;64(5):991-9; discussion 999-1000. doi: 10.1227/01.NEU.0000341905.57162.1D. Neurosurgery. 2009. PMID: 19404159
-
The role of the serotonergic system in locomotor recovery after spinal cord injury.Front Neural Circuits. 2015 Feb 9;8:151. doi: 10.3389/fncir.2014.00151. eCollection 2014. Front Neural Circuits. 2015. PMID: 25709569 Free PMC article. Review.
-
Promoting axonal myelination for improving neurological recovery in spinal cord injury.J Neurotrauma. 2009 Oct;26(10):1847-56. doi: 10.1089/neu.2008.0551. J Neurotrauma. 2009. PMID: 19785544 Review.
Cited by
-
Erythropoietin as a Neuroprotective Molecule: An Overview of Its Therapeutic Potential in Neurodegenerative Diseases.ASN Neuro. 2019 Jan-Dec;11:1759091419871420. doi: 10.1177/1759091419871420. ASN Neuro. 2019. PMID: 31450955 Free PMC article. Review.
-
HuR interacts with lincBRN1a and lincBRN1b during neuronal stem cells differentiation.RNA Biol. 2019 Oct;16(10):1471-1485. doi: 10.1080/15476286.2019.1637698. Epub 2019 Jul 26. RNA Biol. 2019. PMID: 31345103 Free PMC article.
-
Counteracting neuroinflammation in experimental Parkinson's disease favors recovery of function: effects of Er-NPCs administration.J Neuroinflammation. 2018 Nov 30;15(1):333. doi: 10.1186/s12974-018-1375-2. J Neuroinflammation. 2018. PMID: 30501635 Free PMC article.
-
Plasma Erythropoietin, IL-17A, and IFNγ as Potential Biomarkers of Motor Function Recovery in a Canine Model of Spinal Cord Injury.J Mol Neurosci. 2020 Nov;70(11):1821-1828. doi: 10.1007/s12031-020-01575-y. Epub 2020 May 16. J Mol Neurosci. 2020. PMID: 32418163 Free PMC article.
-
Wheel Running Improves Motor Function and Spinal Cord Plasticity in Mice With Genetic Absence of the Corticospinal Tract.Front Cell Neurosci. 2019 Mar 19;13:106. doi: 10.3389/fncel.2019.00106. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30941019 Free PMC article.
References
-
- Abematsu M., Smith I., & Nakashima K. (2006). Mechanisms of neural stem cell fate determination: Extracellular cues and intracellular programs. Curr. Stem Cell Research and Therapy, 1, 267–277. - PubMed
-
- Ahuja C.S., Nori S., Tetreault L., Wilson J., Kwon B., Harrop J., Choi D., & Fehlings M.G. (2017). Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery, 80(3S):S9–S22. doi: 10.1093/neuros/nyw080 - DOI - PubMed
-
- Aigner L., Arber S., Kapfhammer J.P., Laux T., Schneider C., Botteri F., Brenner H.R., & Caroni P. (1995). Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell, 83, 269–278. - PubMed
-
- Ballermann M., & Fouad K. (2006). Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. European Journal of Neuroscience, 8, 1988–1996. doi: 10.1111/j.1460-9568.2006.04726.x - DOI - PubMed
-
- Basso D.M., Fisher L.C., Anderson A.J., Jakeman L.B., Mctigue D.M., & Popovich P.G. (2006). Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. Journal of Neurotrauma, 23, 635–659. doi: 10.1089/neu.2006.23.635 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

