Development of mannose functionalized dendrimeric nanoparticles for targeted delivery to macrophages: use of this platform to modulate atherosclerosis
- PMID: 29172034
- PMCID: PMC6198660
- DOI: 10.1016/j.trsl.2017.10.008
Development of mannose functionalized dendrimeric nanoparticles for targeted delivery to macrophages: use of this platform to modulate atherosclerosis
Abstract
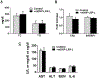
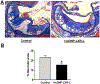
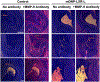
Dysfunctional macrophages underlie the development of several diseases including atherosclerosis where accumulation of cholesteryl esters and persistent inflammation are 2 of the critical macrophage processes that regulate the progression as well as stability of atherosclerotic plaques. Ligand-dependent activation of liver-x-receptor (LXR) not only enhances mobilization of stored cholesteryl ester but also exerts anti-inflammatory effects mediated via trans-repression of proinflammatory transcription factor nuclear factor kappa B. However, increased hepatic lipogenesis by systemic administration of LXR ligands (LXR-L) has precluded their therapeutic use. The objective of the present study was to devise a strategy to selectively deliver LXR-L to atherosclerotic plaque-associated macrophages while limiting hepatic uptake. Mannose-functionalized dendrimeric nanoparticles (mDNP) were synthesized to facilitate active uptake via the mannose receptor expressed exclusively by macrophages using polyamidoamine dendrimer. Terminal amine groups were used to conjugate mannose and LXR-L T091317 via polyethylene glycol spacers. mDNP-LXR-L was effectively taken up by macrophages (and not by hepatocytes), increased expression of LXR target genes (ABCA1/ABCG1), and enhanced cholesterol efflux. When administered intravenously to LDLR-/- mice with established plaques, significant accumulation of fluorescently labeled mDNP-LXR-L was seen in atherosclerotic plaque-associated macrophages. Four weekly injections of mDNP-LXR-L led to significant reduction in atherosclerotic plaque progression, plaque necrosis, and plaque inflammation as assessed by expression of nuclear factor kappa B target gene matrix metalloproteinase 9; no increase in hepatic lipogenic genes or plasma lipids was observed. These studies validate the development of a macrophage-specific delivery platform for the delivery of anti-atherosclerotic agents directly to the plaque-associated macrophages to attenuate plaque burden.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Nanoparticle-based "Two-pronged" approach to regress atherosclerosis by simultaneous modulation of cholesterol influx and efflux.Biomaterials. 2020 Nov;260:120333. doi: 10.1016/j.biomaterials.2020.120333. Epub 2020 Aug 15. Biomaterials. 2020. PMID: 32853832 Free PMC article.
-
Activation of liver X receptor decreases atherosclerosis in Ldlr⁻/⁻ mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells.Arterioscler Thromb Vasc Biol. 2014 Feb;34(2):279-84. doi: 10.1161/ATVBAHA.113.302781. Epub 2013 Dec 5. Arterioscler Thromb Vasc Biol. 2014. PMID: 24311381 Free PMC article.
-
Nanoparticles containing a liver X receptor agonist inhibit inflammation and atherosclerosis.Adv Healthc Mater. 2015 Jan 28;4(2):228-36. doi: 10.1002/adhm.201400337. Epub 2014 Aug 22. Adv Healthc Mater. 2015. PMID: 25156796 Free PMC article.
-
Liver X receptor: a potential target in the treatment of atherosclerosis.Expert Opin Ther Targets. 2022 Jul;26(7):645-658. doi: 10.1080/14728222.2022.2117610. Epub 2022 Sep 5. Expert Opin Ther Targets. 2022. PMID: 36003057 Review.
-
Non-steroidal LXR agonists; an emerging therapeutic strategy for the treatment of atherosclerosis.Recent Pat Cardiovasc Drug Discov. 2006 Jan;1(1):21-46. doi: 10.2174/157489006775244245. Recent Pat Cardiovasc Drug Discov. 2006. PMID: 18221072 Review.
Cited by
-
Advancements in Macrophage-Targeted Drug Delivery for Effective Disease Management.Int J Nanomedicine. 2023 Nov 23;18:6915-6940. doi: 10.2147/IJN.S430877. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38026516 Free PMC article. Review.
-
Macrophage Phenotyping in Atherosclerosis by Proteomics.Int J Mol Sci. 2023 Jan 30;24(3):2613. doi: 10.3390/ijms24032613. Int J Mol Sci. 2023. PMID: 36768933 Free PMC article. Review.
-
Platinum-Based Nanovectors Engineered with Immuno-Modulating Adjuvant for Inhibiting Tumor growth and Promoting Immunity.Theranostics. 2018 Apr 18;8(11):2974-2987. doi: 10.7150/thno.24110. eCollection 2018. Theranostics. 2018. PMID: 29896297 Free PMC article.
-
Inhalable Formulation Based on Lipid-Polymer Hybrid Nanoparticles for the Macrophage Targeted Delivery of Roflumilast.Biomacromolecules. 2022 Aug 8;23(8):3439-3451. doi: 10.1021/acs.biomac.2c00576. Epub 2022 Jul 28. Biomacromolecules. 2022. PMID: 35899612 Free PMC article.
-
Lipid-laden foam cells in the pathology of atherosclerosis: shedding light on new therapeutic targets.Expert Opin Ther Targets. 2023 Jul-Dec;27(12):1231-1245. doi: 10.1080/14728222.2023.2288272. Epub 2023 Dec 30. Expert Opin Ther Targets. 2023. PMID: 38009300 Free PMC article. Review.
References
-
- Martinez-Pomares L The mannose receptor. J Leukoc Biol 2012;92:1177–86. - PubMed
-
- Miyanishi M, Tada K, Koike M, et al. Identification of tim4 as a phosphatidylserine receptor. Nature 2007;450:435–9. - PubMed
-
- Park D, Tosello-Trampont A-C, Elliott MR, et al. Bai1 is an engulfment receptor for apoptotic cells upstream of the elmo/dock180/rac module. Nature 2007;450:430–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

