A phase I, randomized, controlled, dose-ranging study of investigational acellular pertussis (aP) and reduced tetanus-diphtheria-acellular pertussis (TdaP) booster vaccines in adults
- PMID: 29172945
- PMCID: PMC5791588
- DOI: 10.1080/21645515.2017.1385686
A phase I, randomized, controlled, dose-ranging study of investigational acellular pertussis (aP) and reduced tetanus-diphtheria-acellular pertussis (TdaP) booster vaccines in adults
Abstract
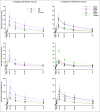
Despite high vaccination coverage worldwide, pertussis has re-emerged in many countries. This randomized, controlled, observer-blind phase I study and extension study in Belgium (March 2012-June 2015) assessed safety and immunogenicity of investigational acellular pertussis vaccines containing genetically detoxified pertussis toxin (PT) (NCT01529645; NCT02382913). 420 healthy adults (average age: 26.8 ± 5.5 years, 60% female) were randomized to 1 of 10 vaccine groups: 3 investigational aP vaccines (containing pertussis antigens PT, filamentous hemagglutinin [FHA] and pertactin [PRN] at different dosages), 6 investigational TdaP (additionally containing tetanus toxoid [TT] and diphtheria toxoid [DT]), and 1 TdaP comparator containing chemically inactivated PT. Antibody responses were evaluated on days 1, 8, 30, 180, 365, and approximately 3 years post-booster vaccination. Cell-mediated immune responses and PT neutralization were evaluated in a subset of participants in pre-selected groups. Local and systemic adverse events (AEs), and unsolicited AEs were collected through day 7 and 30, respectively; serious AEs and AEs leading to study withdrawal were collected through day 365 post-vaccination. Antibody responses against pertussis antigens peaked at day 30 post-vaccination and then declined but remained above baseline level at approximately 3 years post-vaccination. Responses to FHA and PRN were correlated to antigen dose. Antibody responses specific to PT, toxin neutralization activity and persistence induced by investigational formulations were similar or significantly higher than the licensed vaccine, despite lower PT doses. Of 15 serious AEs, none were considered vaccination-related; 1 led to study withdrawal (premature labor, day 364; aP4 group). This study confirmed the potential benefits of genetically detoxified PT antigen. All investigational study formulations were well tolerated.
Keywords: adults; genetically detoxified PT; immunogenicity; persistence; pertussis; safety.
Figures






References
-
- Centers for Disease Control and Prevention. Pertussis (Whooping Cough). Complications; [accessed 1 August 2016]. http://www.cdc.gov/pertussis/about/complications.html
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
