A Comparison of Brand and Biosimilar Granulocyte-Colony Stimulating Factors for Prophylaxis of Chemotherapy-Induced Febrile Neutropenia
- PMID: 29172983
- PMCID: PMC10398039
- DOI: 10.18553/jmcp.2017.23.12.1221
A Comparison of Brand and Biosimilar Granulocyte-Colony Stimulating Factors for Prophylaxis of Chemotherapy-Induced Febrile Neutropenia
Abstract
Background: Filgrastim-sndz, a granulocyte-colony stimulating factor (G-CSF), was introduced as a biosimilar to filgrastim in 2015, but real-world comparative effectiveness for filgrastim versus filgrastim-sndz has not been reported to date.
Objectives: To (a) compare the incidence of febrile neutropenia for patients taking filgrastim versus those taking filgrastim-sndz and (b) compare the incidence of a potential serious adverse event for filgrastim versus filgrastim-sndz.
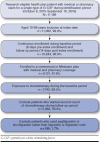
Methods: This retrospective cohort study identified patients receiving a G-CSF following chemotherapy, using administrative claims from the Humana Research Database. Patients enrolled in a Medicare Advantage Prescription Drug plan with a claim for a G-CSF from October 1, 2015, through September 30, 2016, were identified. G-CSF use had to occur within 6 days of exposure to chemotherapy and without any subsequent chemotherapy within 14 days after G-CSF use. Febrile neutropenia requiring hospitalization was defined as hospitalization within 14 days after G-CSF use with (a) diagnosis of infection and/or neutropenia (broad definition) or (b) infection and neutropenia diagnoses (narrow definition). Serious adverse drug events (spleen rupture, acute respiratory syndrome, serious allergic reactions, capillary leak syndrome, thrombocytopenia, leukocytosis, cutaneous vasculitis, or bones and muscle ache) were also identified within 14 days after G-CSF use. An incidence difference of < 1% with 90% CI crossing zero qualified as support for noninferiority. Two-tailed chi-square tests were also used to investigate differences.
Results: A total of 88 filgrastim and 101 filgrastim-sndz patients were identified. Filgrastim and filgrastim-sndz met the criteria for noninferiority based on an incidence difference of -0.6% (90% CI = -5.1%-4.0%; P = 0.84) for the broad definition of febrile neutropenia and a difference of -0.8% (90% CI = -3.8%-2.1%; P = 0.64) for the narrow definition. For the analysis of serious adverse events, an incidence difference of -2.5% (90% CI = -7.5%-2.5%; P = 0.42) for filgrastim compared with filgrastim-sndz was not sufficient to establish noninferiority.
Conclusions: This study is one of the first analyses of real-world evidence regarding the noninferiority of filgrastim and filgrastim-sndz. The study results support noninferiority of filgrastim and filgrastim-sndz for prevention of febrile neutropenia requiring hospitalization. While noninferiority for serious adverse events was not supported, there was also no statistically significant difference between filgrastim and filgrastim-sndz. The study's small sample size could have limited the analysis of the relatively rare outcomes of febrile neutropenia requiring hospitalization and serious adverse events. A study including a larger numbers of patients taking filgrastim or filgrastim-sndz could provide additional insights.
Disclosures: This study received no outside funding. Douglas, Kennedy, and Slabaugh were employees of Humana Pharmacy Solutions at the time the study was conducted. Bowe, Schwab, and Lane were employees of Comprehensive Health Insights, a wholly owned subsidiary of Humana, at the time the study was conducted. Study concept and design were contributed by Douglas, Kennedy, Schwab, and Lane, along with Slabaugh and Bowe. Bowe took the lead in data collection, assisted by Schwab, and data interpretation was performed by Schwab, along with the other authors. The manuscript was written by Schwab, Lane, and Douglas and revised by Kennedy, Slabaugh, and Bowe, along with Schwab, Lane, and Douglas.
Conflict of interest statement
This study received no outside funding. Douglas, Kennedy, and Slabaugh were employees of Humana Pharmacy Solutions at the time the study was conducted. Bowe, Schwab, and Lane were employees of Comprehensive Health Insights, a wholly owned subsidiary of Humana, at the time the study was conducted.
Study concept and design were contributed by Douglas, Kennedy, Schwab, and Lane, along with Slabaugh and Bowe. Bowe took the lead in data collection, assisted by Schwab, and data interpretation was performed by Schwab, along with the other authors. The manuscript was written by Schwab, Lane, and Douglas and revised by Kennedy, Slabaugh, and Bowe, along with Schwab, Lane, and Douglas.
References
-
- Biologic Price Competition and Innovation Act. Title VII, Subtitle A of H.R. 3590. 111th Congress (2009-2010). Enacted. Available at: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformat.... Accessed October 31, 2017.
-
- U.S. Food and Drug Administration. FDA approves first biosimilar product Zarxio. FDA news release. March 6, 2015. Available at: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm436648.htm. Accessed October 31, 2017.
-
- Zarxio (filgrastim-sndz) injection, for subcutaneous or intravenous use. Sandoz. Revised February 2017. Available at: http://www.zarxio.com/globalassets/zarxio2/resources/materials/S-Zarxio-.... Accessed November 8, 2017.
-
- Neupogen (filgrastim) injection, for subcutaneous or intravenous use. Amgen. Revised July 2015. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5186lbl.... Accessed October 31, 2017.
-
- Granix (tbo-filgrastim) injection, for subcutaneous use. Sicor Biotech UAB. Revised December 2014. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125294s035lbl.pdf. Accessed October 31, 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous