Treatment of Children with Persistent and Chronic Idiopathic Thrombocytopenic Purpura: 4 Infusions of Rituximab and Three 4-Day Cycles of Dexamethasone
- PMID: 29173312
- PMCID: PMC6020036
- DOI: 10.1016/j.jpeds.2017.08.036
Treatment of Children with Persistent and Chronic Idiopathic Thrombocytopenic Purpura: 4 Infusions of Rituximab and Three 4-Day Cycles of Dexamethasone
Abstract
Objectives: To assess initial and long-term outcome of children with persistent/chronic idiopathic thrombocytopenic purpura (ITP) treated with 4 infusions of rituximab and three 4-day cycles of dexamethasone (4R+3Dex) including cohorts with most benefit and/or treatment associated toxicity.
Study design: All pediatric patients with ITP at Weill-Cornell who received 4R+3Dex were included in this retrospective study. Duration was median time from first rituximab infusion to treatment failure. Patient cohort included 33 children ages 1-18 years with persistent/chronic ITP; 19 were female, 10 of whom were adolescents. Every patient had failed more than 1 and usually several ITP treatments.
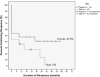
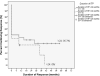
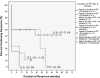
Results: Children were treated with rituximab, 375 mg/m2 weekly for 4 weeks and three 4-day courses of dexamethasone 28 mg/m2 (40 mg max). Average age of nonresponders was 7.75 years, and initial responders averaged 12.7 years (P = .0073); 30% maintained continuing response at 60 months or last check-up. Eight of the 10 patients who underwent remission were female with ITP <24 months prior to initiating 4R+3Dex. All responding male patients except 2 relapsed.
Conclusions: Durable unmaintained ITP remission after 4R+3Dex was seen almost exclusively in female adolescents with <24 months duration of ITP. This provides a new therapeutic paradigm for a subpopulation with hard-to-treat chronic ITP. The pathophysiology of ITP underlying this distinction requires further elucidation.
Keywords: autoimmune disease; immune therapy; platelets; thrombocytopenia.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Combination immune-based therapy for chronic ITP.J Pediatr. 2017 Dec;191:1. doi: 10.1016/j.jpeds.2017.10.019. J Pediatr. 2017. PMID: 29173291 No abstract available.
Similar articles
-
Gender and duration of disease differentiate responses to rituximab-dexamethasone therapy in adults with immune thrombocytopenia.Am J Hematol. 2016 Sep;91(9):907-11. doi: 10.1002/ajh.24434. Epub 2016 Jun 20. Am J Hematol. 2016. PMID: 27220625
-
Rituximab in auto-immune haemolytic anaemia and immune thrombocytopenic purpura: a Belgian retrospective multicentric study.J Intern Med. 2009 Nov;266(5):484-91. doi: 10.1111/j.1365-2796.2009.02126.x. Epub 2009 Apr 27. J Intern Med. 2009. PMID: 19549092
-
Randomized study of IVIg and high-dose dexamethasone therapy for children with chronic idiopathic thrombocytopenic purpura.J Pediatr Hematol Oncol. 2003 Feb;25(2):139-44. doi: 10.1097/00043426-200302000-00011. J Pediatr Hematol Oncol. 2003. PMID: 12571466 Clinical Trial.
-
Rituximab alleviates pediatric systemic lupus erythematosus associated refractory immune thrombocytopenia: a case-based review.Immunol Res. 2024 Jun;72(3):503-511. doi: 10.1007/s12026-024-09454-z. Epub 2024 Jan 27. Immunol Res. 2024. PMID: 38279058 Review.
-
Efficacy and Safety of the Combination Treatment of Rituximab and Dexamethasone for Adults with Primary Immune Thrombocytopenia (ITP): A Meta-Analysis.Biomed Res Int. 2018 Dec 12;2018:1316096. doi: 10.1155/2018/1316096. eCollection 2018. Biomed Res Int. 2018. PMID: 30648105 Free PMC article. Review.
Cited by
-
Rituximab in the treatment of immune thrombocytopenia: what is the role of this agent in 2019?Haematologica. 2019 Jun;104(6):1124-1135. doi: 10.3324/haematol.2019.218883. Epub 2019 May 24. Haematologica. 2019. PMID: 31126963 Free PMC article. Review.
-
Efficacy and safety of rituximab for minors with immune thrombocytopenia: a systematic review and meta-analysis.J Int Med Res. 2020 Oct;48(10):300060520962348. doi: 10.1177/0300060520962348. J Int Med Res. 2020. PMID: 33115308 Free PMC article.
-
Therapeutic effects of rituximab combined with cyclophosphamide on refractory idiopathic thrombocytopenic purpura.Exp Ther Med. 2019 Mar;17(3):2137-2142. doi: 10.3892/etm.2019.7196. Epub 2019 Jan 23. Exp Ther Med. 2019. PMID: 30867701 Free PMC article.
-
Adjuvant rituximab, a potential treatment for the young patient with Graves' hyperthyroidism (RiGD): study protocol for a single-arm, single-stage, phase II trial.BMJ Open. 2019 Jan 21;9(1):e024705. doi: 10.1136/bmjopen-2018-024705. BMJ Open. 2019. PMID: 30670519 Free PMC article.
-
Advances in management of pediatric chronic immune thrombocytopenia: a narrative review.J Yeungnam Med Sci. 2023 Jul;40(3):241-246. doi: 10.12701/jyms.2022.00745. Epub 2023 Jan 9. J Yeungnam Med Sci. 2023. PMID: 36617702 Free PMC article.
References
-
- Olson B, Andersson PO, Jernas M, Jacobsson S, Carlsson B, Carlsson LM, et al. T-cell mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9:1123–4. - PubMed
-
- McKenzie CG, Guo L, Freedman J, Semple JW. Cellular immune dysfunction in immune thrombocytopenia (ITP) Br J Haematol. 2013;163:10–23. - PubMed
-
- Chang M, Nakagawa PA, Williams SA, Schwartz MR, Imfeld KL, Buzby JS, et al. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood. 2003;102:887–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

