The effect of cool water pack preparation on vaccine vial temperatures in refrigerators
- PMID: 29174313
- PMCID: PMC5736983
- DOI: 10.1016/j.vaccine.2017.11.024
The effect of cool water pack preparation on vaccine vial temperatures in refrigerators
Abstract
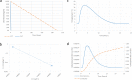
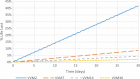
Cool water packs are a useful alternative to ice packs for preventing unintentional freezing of vaccines during outreach in some situations. Current guidelines recommend the use of a separate refrigerator for cooling water packs from ambient temperatures to prevent possible heat degradation of adjacent vaccine vials. To investigate whether this additional equipment is necessary, we measured the temperatures that vaccine vials were exposed to when warm water packs were placed next to vials in a refrigerator. We then calculated the effect of repeated vial exposure to those temperatures on vaccine vial monitor status to estimate the impact to the vaccine. Vials were tested in a variety of configurations, varying the number and locations of vials and water packs in the refrigerator. The calculated average percentage life lost during a month of repeated warming ranged from 20.0% to 30.3% for a category 2 (least stable) vaccine vial monitor and from 3.8% to 6.0% for a category 7 (moderate stability) vaccine vial monitor, compared to 17.0% for category 2 vaccine vial monitors and 3.1% for category 7 vaccine vial monitors at a constant 5 °C. The number of vials, number of water packs, and locations of each impacted vial warming and therefore percentage life lost, but the vaccine vial monitor category had a higher impact on the average percentage life lost than any of the other parameters. The results suggest that damage to vaccines from repeated warming over the course of a month is not certain and that cooling water packs in a refrigerator where vaccines are being stored may be a useful practice if safe procedures are established.
Keywords: Cold chain; Cool water pack; Freeze-sensitive vaccine; Supply chain; Vaccine; Vaccine vial monitor.
Copyright © 2017 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures



Similar articles
-
Use of cool water packs to prevent freezing during vaccine transportation at the country level.PDA J Pharm Sci Technol. 2009 Jan-Feb;63(1):11-26. PDA J Pharm Sci Technol. 2009. PMID: 19455939
-
Relationship between vaccine vial monitors and cold chain infrastructure in a rural district of India.Rural Remote Health. 2007 Jan-Mar;7(1):617. Epub 2007 Feb 9. Rural Remote Health. 2007. PMID: 17288508
-
Hepatitis B vaccine freezing in the Indonesian cold chain: evidence and solutions.Bull World Health Organ. 2004 Feb;82(2):99-105. Epub 2004 Mar 16. Bull World Health Organ. 2004. PMID: 15042231 Free PMC article.
-
Tools and approaches to ensure quality of vaccines throughout the cold chain.Expert Rev Vaccines. 2014 Jul;13(7):843-54. doi: 10.1586/14760584.2014.923761. Epub 2014 May 28. Expert Rev Vaccines. 2014. PMID: 24865112 Free PMC article. Review.
-
Is freezing in the vaccine cold chain an ongoing issue? A literature review.Vaccine. 2017 Apr 19;35(17):2127-2133. doi: 10.1016/j.vaccine.2016.09.070. Vaccine. 2017. PMID: 28364920 Review.
Cited by
-
[Evaluation on monitoring effect of the electronic vaccine vial monitor label].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021 Feb 25;38(1):154-160. doi: 10.7507/1001-5515.202011038. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021. PMID: 33899440 Free PMC article. Chinese.
-
Using long-range freeze-preventive vaccine carriers in Nepal: A study of equipment performance, acceptability, systems fit, and cost.Vaccine X. 2022 Feb 11;10:100146. doi: 10.1016/j.jvacx.2022.100146. eCollection 2022 Apr. Vaccine X. 2022. PMID: 35243322 Free PMC article.
-
Evaluation of precipitation time of the aluminum salts adsorbed potentially frozen vaccines used in the Polish National Immunization Schedule for their pre-qualification before the administration.Clin Exp Vaccine Res. 2022 May;11(2):155-162. doi: 10.7774/cevr.2022.11.2.155. Epub 2022 May 31. Clin Exp Vaccine Res. 2022. PMID: 35799879 Free PMC article.
References
-
- Hanson C.M., George A.M., Sawadogo A., Schreiber B. Is freezing in the vaccine cold chain an ongoing issue? A literature review. Vaccine. 2017;35(17):2127–2133. - PubMed
-
- World Health Organization (WHO). A guide to introducing inactivated polio vaccine—based on the polio eradication and strategic endgame strategic plan 2013–2018. Geneva: WHO; 2016. Available from: <http://www.who.int/immunization/diseases/poliomyelitis/endgame_objective...> [accessed 8 May 2017].
-
- McLean A.A., Shaw R. Hepatitis B virus vaccine. Ann Intern Med. 1982;97(3):451. - PubMed
-
- Davaalkham D., Ojima T., Wiersma S., Lkhagvasuren T., Nymadawa P., Uehara R. Administration of hepatitis B vaccine in winter as a significant predictor of the poor effectiveness of vaccination in rural Mongolia: evidence from a nationwide survey. J Epidemiol Commun Health. 2007;61(7):578–584. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

