Injury Induces Endogenous Reprogramming and Dedifferentiation of Neuronal Progenitors to Multipotency
- PMID: 29174332
- PMCID: PMC5722700
- DOI: 10.1016/j.stem.2017.09.008
Injury Induces Endogenous Reprogramming and Dedifferentiation of Neuronal Progenitors to Multipotency
Abstract
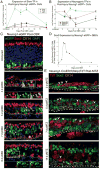
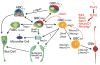
Adult neurogenesis in the olfactory epithelium is often depicted as a unidirectional pathway during homeostasis and repair. We challenge the unidirectionality of this model by showing that epithelial injury unlocks the potential for Ascl1+ progenitors and Neurog1+ specified neuronal precursors to dedifferentiate into multipotent stem/progenitor cells that contribute significantly to tissue regeneration in the murine olfactory epithelium (OE). We characterize these dedifferentiating cells using several lineage-tracing strains and single-cell mRNA-seq, and we show that Sox2 is required for initiating dedifferentiation and that inhibition of Ezh2 promotes multipotent progenitor expansion. These results suggest that the apparent hierarchy of neuronal differentiation is not irreversible and that lineage commitment can be overridden following severe tissue injury. We elucidate a previously unappreciated pathway for endogenous tissue repair by a highly regenerative neuroepithelium and introduce a system to study the mechanisms underlying plasticity in the OE that can be adapted for other tissues.
Keywords: adult neurogenesis; dedifferentiation; olfactory epithelium; reprogramming.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Plasticity in Olfactory Epithelium: Is It a Sniffer or Shape Shifter?Cell Stem Cell. 2017 Dec 7;21(6):707-708. doi: 10.1016/j.stem.2017.11.014. Cell Stem Cell. 2017. PMID: 29220660
References
-
- Cau E, Gradwohl G, Fode C, Guillemot F. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

