Src family kinases (SFKs) and cell polarity in the testis
- PMID: 29174914
- PMCID: PMC5988912
- DOI: 10.1016/j.semcdb.2017.11.024
Src family kinases (SFKs) and cell polarity in the testis
Abstract
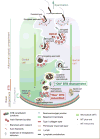
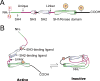
Non-receptor Src family kinases (SFKs), most notably c-Src and c-Yes, are recently shown to be expressed by Sertoli and/or germ cells in adult rat testes. Studies have shown that SFKs are involved in modulating the cell cytoskeletal function, and involved in endocytic vesicle-mediated protein endocytosis, transcytosis and/or recycling as well as intracellular protein degradation events. Furthermore, a knockdown to SFKs, in particular c-Yes, has shown to induce defects in spermatid polarity. These findings, coupled with emerging evidence in the field, thus prompt us to critically evaluate them to put forth a developing concept regarding the role of SFKs and cell polarity, which will become a basis to design experiments for future investigations.
Keywords: Cell polarity; Sertoli cells; Spermatids; Spermatogenesis; Src family kinases; Testis; c-Src; c-Yes.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


Similar articles
-
Cell polarity and planar cell polarity (PCP) in spermatogenesis.Semin Cell Dev Biol. 2018 Sep;81:71-77. doi: 10.1016/j.semcdb.2017.09.008. Epub 2017 Sep 29. Semin Cell Dev Biol. 2018. PMID: 28923514 Free PMC article. Review.
-
C-Src and c-Yes are two unlikely partners of spermatogenesis and their roles in blood-testis barrier dynamics.Adv Exp Med Biol. 2012;763:295-317. doi: 10.1007/978-1-4614-4711-5_15. Adv Exp Med Biol. 2012. PMID: 23397631 Free PMC article.
-
Regulation of spermatid polarity by the actin- and microtubule (MT)-based cytoskeletons.Semin Cell Dev Biol. 2018 Sep;81:88-96. doi: 10.1016/j.semcdb.2018.01.013. Epub 2018 Jul 12. Semin Cell Dev Biol. 2018. PMID: 29410206 Free PMC article. Review.
-
Cell polarity and cytoskeletons-Lesson from the testis.Semin Cell Dev Biol. 2018 Sep;81:21-32. doi: 10.1016/j.semcdb.2017.09.037. Epub 2017 Oct 6. Semin Cell Dev Biol. 2018. PMID: 28965865 Free PMC article. Review.
-
Planar Cell Polarity (PCP) Protein Vangl2 Regulates Ectoplasmic Specialization Dynamics via Its Effects on Actin Microfilaments in the Testes of Male Rats.Endocrinology. 2016 May;157(5):2140-59. doi: 10.1210/en.2015-1987. Epub 2016 Mar 18. Endocrinology. 2016. PMID: 26990065 Free PMC article.
Cited by
-
α5 integrin regulates hepatic tight junctions through SRC-TET1-mediated DNA hydroxymethylation.iScience. 2022 Nov 17;25(12):105611. doi: 10.1016/j.isci.2022.105611. eCollection 2022 Dec 22. iScience. 2022. PMID: 36465132 Free PMC article.
-
Proto-oncogene tyrosine-protein kinase SRC (Src) inhibition in microglia relieves neuroinflammation in neuropathic pain mouse models.Bioengineered. 2021 Dec;12(2):11390-11398. doi: 10.1080/21655979.2021.2008694. Bioengineered. 2021. PMID: 34851237 Free PMC article.
-
Exploring the evolving function of soluble intercellular adhesion molecule-1 in junction dynamics during spermatogenesis.Front Endocrinol (Lausanne). 2024 Jan 8;14:1281812. doi: 10.3389/fendo.2023.1281812. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38260159 Free PMC article. Review.
References
-
- Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

