PTEN-Dependent Stabilization of MTSS1 Inhibits Metastatic Phenotype in Pancreatic Ductal Adenocarcinoma
- PMID: 29175021
- PMCID: PMC5714254
- DOI: 10.1016/j.neo.2017.10.004
PTEN-Dependent Stabilization of MTSS1 Inhibits Metastatic Phenotype in Pancreatic Ductal Adenocarcinoma
Abstract
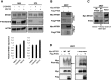
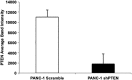
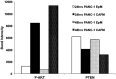
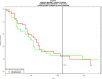
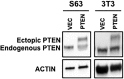
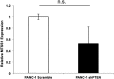
Pancreatic ductal adenocarcinoma (PDAC) presents at metastatic stage in over 50% of patients. With a survival rate of just 2.7% for patients presenting with distant disease, it is imperative to uncover novel mechanisms capable of suppressing metastasis in PDAC. Previously, we reported that the loss of metastasis suppressor protein 1 (MTSS1) in PDAC cells results in significant increase in cellular migration and invasion. Conversely, we also found that overexpressing MTSS1 in metastatic PDAC cell lines corresponds with not only decreased metastatic phenotype, but also greater overall survival. While it is known that MTSS1 is downregulated in late-stage PDAC, the mechanism behind that loss has not yet been elucidated. Here, we build off our previous findings to present a novel regulatory mechanism for the stabilization of MTSS1 via the tumor suppressor protein phosphatase and tensin homolog (PTEN). We show that PTEN loss in PDAC cells results in a decrease in MTSS1 expression and increased metastatic potential. Additionally, we demonstrate that PTEN forms a complex with MTSS1 in order to stabilize and protect it from proteasomal degradation. Finally, we show that the inflammatory tumor microenvironment, which makes up over 90% of PDAC tumor bulk, is capable of downregulating PTEN expression through secretion of miRNA-23b, potentially uncovering a novel extrinsic mechanism of MTSS1 regulation. Collectively, these data offer new insight into the role and regulation of MTSS1in suppressing tumor cell invasion and migration and help shed light as to what molecular mechanisms could be leading to early cell dissemination in PDAC.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






Similar articles
-
MTA2-mediated inhibition of PTEN leads to pancreatic ductal adenocarcinoma carcinogenicity.Cell Death Dis. 2019 Feb 27;10(3):206. doi: 10.1038/s41419-019-1424-5. Cell Death Dis. 2019. PMID: 30814496 Free PMC article.
-
Loss of MTSS1 results in increased metastatic potential in pancreatic cancer.Oncotarget. 2017 Mar 7;8(10):16473-16487. doi: 10.18632/oncotarget.14869. Oncotarget. 2017. PMID: 28146435 Free PMC article.
-
Metastasis suppressor protein 1 regulated by PTEN suppresses invasion, migration, and EMT of gastric carcinoma by inactivating PI3K/AKT signaling.J Cell Biochem. 2019 Mar;120(3):3447-3454. doi: 10.1002/jcb.27618. Epub 2018 Sep 23. J Cell Biochem. 2019. PMID: 30246429
-
The mechanisms behind the dual role of long non-coding RNA (lncRNA) metastasis suppressor-1 in human tumors: Shedding light on the molecular mechanisms.Pathol Res Pract. 2024 Apr;256:155189. doi: 10.1016/j.prp.2024.155189. Epub 2024 Feb 16. Pathol Res Pract. 2024. PMID: 38452581 Review.
-
Metastasis Suppressor Genes in Pancreatic Cancer: An Update.Pancreas. 2021 Aug 1;50(7):923-932. doi: 10.1097/MPA.0000000000001853. Pancreas. 2021. PMID: 34643607 Review.
Cited by
-
Computational analysis of the evolutionarily conserved Missing In Metastasis/Metastasis Suppressor 1 gene predicts novel interactions, regulatory regions and transcriptional control.Sci Rep. 2019 Mar 11;9(1):4155. doi: 10.1038/s41598-019-40697-1. Sci Rep. 2019. PMID: 30858428 Free PMC article.
-
Stable Isotopes for Tracing Cardiac Metabolism in Diseases.Front Cardiovasc Med. 2021 Nov 11;8:734364. doi: 10.3389/fcvm.2021.734364. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34859064 Free PMC article. Review.
-
PTEN regulates invasiveness in pancreatic neuroendocrine tumors through DUSP19-mediated VEGFR3 dephosphorylation.J Biomed Sci. 2022 Nov 6;29(1):92. doi: 10.1186/s12929-022-00875-2. J Biomed Sci. 2022. PMID: 36336681 Free PMC article.
-
MTA2-mediated inhibition of PTEN leads to pancreatic ductal adenocarcinoma carcinogenicity.Cell Death Dis. 2019 Feb 27;10(3):206. doi: 10.1038/s41419-019-1424-5. Cell Death Dis. 2019. PMID: 30814496 Free PMC article.
-
Metastasis suppressor 1 controls osteoblast differentiation and bone homeostasis through regulating Src-Wnt/β-catenin signaling.Cell Mol Life Sci. 2022 Jan 30;79(2):107. doi: 10.1007/s00018-022-04147-y. Cell Mol Life Sci. 2022. PMID: 35094173 Free PMC article.
References
-
- Society. AC . The Society; Atlanta, GA: 2010. Cancer Facts and Figures; p. v.
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66(1):7–30. - PubMed
-
- Loncle C, Bonjoch L, Folch-Puy E, Lopez-Millan MB, Lac S, Molejon MI, Chuluyan E, Cordelier P, Dubus P, Lomberk G. IL17 Functions through the Novel REG3beta-JAK2-STAT3 Inflammatory Pathway to Promote the Transition from Chronic Pancreatitis to Pancreatic Cancer. Cancer Res. 2015;75(22):4852–4862. - PMC - PubMed
-
- Loncle C, Molejon MI, Lac S, Tellechea JI, Lomberk G, Gramatica L, Fernandez Zapico MF, Dusetti N, Urrutia R, Iovanna JL. The pancreatitis-associated protein VMP1, a key regulator of inducible autophagy, promotes Kras(G12D)-mediated pancreatic cancer initiation. Cell Death Dis. 2016;7:e2295. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

