Vacuolar protein sorting 13C is a novel lipid droplet protein that inhibits lipolysis in brown adipocytes
- PMID: 29175050
- PMCID: PMC5784322
- DOI: 10.1016/j.molmet.2017.10.014
Vacuolar protein sorting 13C is a novel lipid droplet protein that inhibits lipolysis in brown adipocytes
Abstract
Objective: Brown adipose tissue (BAT) thermogenesis depends on the mobilization and oxidation of fatty acids from intracellular lipid droplets (LD) within brown adipocytes (BAs); however, the identity and function of LD proteins that control BAT lipolysis remain incomplete. Proteomic analysis of mouse BAT subcellular fractions identified vacuolar protein sorting 13C (VPS13C) as a novel LD protein. The aim of this work was to investigate the role of VPS13C on BA LDs.
Methods: Biochemical fractionation and high resolution confocal and immuno-transmission electron microscopy (TEM) were used to determine the subcellular distribution of VPS13C in mouse BAT, white adipose tissue, and BA cell culture. Lentivirus-delivered shRNA was used to determine the role of VPS13C in regulating lipolysis and gene expression in cultured BA cells.
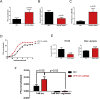
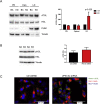
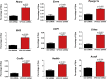
Results: We found that VPS13C is highly expressed in mouse BAT where it is targeted to multilocular LDs in a subspherical subdomain. In inguinal white adipocytes, VPS13C was mainly observed on small LDs and β3-adrenergic stimulation increased VPS13C in this depot. Silencing of VPS13C in cultured BAs decreased LD size and triglyceride content, increased basal free fatty acid release, augmented the expression of thermogenic genes, and enhanced the lipolytic potency and efficacy of isoproterenol. Mechanistically, we found that BA lipolysis required activation of adipose tissue triglyceride lipase (ATGL) and that loss of VPS13C greatly increased the association of ATGL to LDs.
Conclusions: VPS13C is present on BA LDs where is targeted to a distinct subdomain. VPS13C limits the access of ATGL to LD and loss of VPS13C elevates lipolysis and promotes oxidative gene expression.
Keywords: ATGL; Brown adipose tissue; Free fatty acids; Oxidative genes; Perilipin 1; Thermogenesis.
Copyright © 2017 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures

Similar articles
-
Cell death-inducing DNA fragmentation factor A-like effector A and fat-specific protein 27β coordinately control lipid droplet size in brown adipocytes.J Biol Chem. 2017 Jun 30;292(26):10824-10834. doi: 10.1074/jbc.M116.768820. Epub 2017 May 10. J Biol Chem. 2017. PMID: 28490632 Free PMC article.
-
Adipocyte-specific Hypoxia-inducible gene 2 promotes fat deposition and diet-induced insulin resistance.Mol Metab. 2016 Sep 28;5(12):1149-1161. doi: 10.1016/j.molmet.2016.09.009. eCollection 2016 Dec. Mol Metab. 2016. PMID: 27900258 Free PMC article.
-
Lipid droplet remodeling and interaction with mitochondria in mouse brown adipose tissue during cold treatment.Biochim Biophys Acta. 2015 May;1853(5):918-28. doi: 10.1016/j.bbamcr.2015.01.020. Epub 2015 Feb 2. Biochim Biophys Acta. 2015. PMID: 25655664
-
CIDE Family-Mediated Unique Lipid Droplet Morphology in White Adipose Tissue and Brown Adipose Tissue Determines the Adipocyte Energy Metabolism.J Atheroscler Thromb. 2017 Oct 1;24(10):989-998. doi: 10.5551/jat.RV17011. Epub 2017 Sep 5. J Atheroscler Thromb. 2017. PMID: 28883211 Free PMC article. Review.
-
Brown adipose tissue and lipid metabolism.Curr Opin Lipidol. 2018 Jun;29(3):180-185. doi: 10.1097/MOL.0000000000000504. Curr Opin Lipidol. 2018. PMID: 29718003 Review.
Cited by
-
In silico modeling human VPS13 proteins associated with donor and target membranes suggests lipid transfer mechanisms.Proteins. 2023 Apr;91(4):439-455. doi: 10.1002/prot.26446. Epub 2022 Dec 5. Proteins. 2023. PMID: 36404287 Free PMC article.
-
Role of VPS13, a protein with similarity to ATG2, in physiology and disease.Curr Opin Genet Dev. 2020 Dec;65:61-68. doi: 10.1016/j.gde.2020.05.027. Epub 2020 Jun 18. Curr Opin Genet Dev. 2020. PMID: 32563856 Free PMC article. Review.
-
VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites.J Cell Biol. 2018 Oct 1;217(10):3625-3639. doi: 10.1083/jcb.201807019. Epub 2018 Aug 9. J Cell Biol. 2018. PMID: 30093493 Free PMC article.
-
TBC1D1 interacting proteins, VPS13A and VPS13C, regulate GLUT4 homeostasis in C2C12 myotubes.Sci Rep. 2020 Oct 21;10(1):17953. doi: 10.1038/s41598-020-74661-1. Sci Rep. 2020. PMID: 33087848 Free PMC article.
-
VPS13D promotes peroxisome biogenesis.J Cell Biol. 2021 May 3;220(5):e202001188. doi: 10.1083/jcb.202001188. J Cell Biol. 2021. PMID: 33891012 Free PMC article.
References
-
- van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D. Cold-activated brown adipose tissue in healthy men. The New England Journal of Medicine. 2009;360(15):1500–1508. - PubMed
-
- Virtanen K.A., Lidell M.E., Orava J., Heglind M., Westergren R., Niemi T. Functional brown adipose tissue in healthy adults. The New England Journal of Medicine. 2009;360(15):1518–1525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

