Fatty acid oxidation is required for active and quiescent brown adipose tissue maintenance and thermogenic programing
- PMID: 29175051
- PMCID: PMC5784326
- DOI: 10.1016/j.molmet.2017.11.004
Fatty acid oxidation is required for active and quiescent brown adipose tissue maintenance and thermogenic programing
Abstract
Objective: To determine the role of fatty acid oxidation on the cellular, molecular, and physiologic response of brown adipose tissue to disparate paradigms of chronic thermogenic stimulation.
Methods: Mice with an adipose-specific loss of Carnitine Palmitoyltransferase 2 (Cpt2A-/-), that lack mitochondrial long chain fatty acid β-oxidation, were subjected to environmental and pharmacologic interventions known to promote thermogenic programming in adipose tissue.
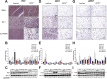
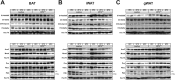
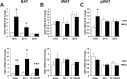
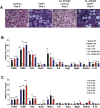
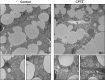
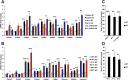
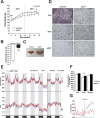
Results: Chronic administration of β3-adrenergic (CL-316243) or thyroid hormone (GC-1) agonists induced a loss of BAT morphology and UCP1 expression in Cpt2A-/- mice. Fatty acid oxidation was also required for the browning of white adipose tissue (WAT) and the induction of UCP1 in WAT. In contrast, chronic cold (15 °C) stimulation induced UCP1 and thermogenic programming in both control and Cpt2A-/- adipose tissue albeit to a lesser extent in Cpt2A-/- mice. However, thermoneutral housing also induced the loss of UCP1 and BAT morphology in Cpt2A-/- mice. Therefore, adipose fatty acid oxidation is required for both the acute agonist-induced activation of BAT and the maintenance of quiescent BAT. Consistent with this data, Cpt2A-/- BAT exhibited increased macrophage infiltration, inflammation and fibrosis irrespective of BAT activation. Finally, obese Cpt2A-/- mice housed at thermoneutrality exhibited a loss of interscapular BAT and were refractory to β3-adrenergic-induced energy expenditure and weight loss.
Conclusion: Mitochondrial long chain fatty acid β-oxidation is critical for the maintenance of the brown adipocyte phenotype both during times of activation and quiescence.
Keywords: Adipose macrophage; Adrenergic signaling; Brown adipose tissue; Cold induced thermogenesis; Fatty acid oxidation.
Copyright © 2017 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures



Similar articles
-
Loss of Adipose Fatty Acid Oxidation Does Not Potentiate Obesity at Thermoneutrality.Cell Rep. 2016 Feb 16;14(6):1308-1316. doi: 10.1016/j.celrep.2016.01.029. Epub 2016 Feb 4. Cell Rep. 2016. PMID: 26854223 Free PMC article.
-
Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation.Cell Rep. 2015 Jan 13;10(2):266-79. doi: 10.1016/j.celrep.2014.12.023. Epub 2015 Jan 8. Cell Rep. 2015. PMID: 25578732 Free PMC article.
-
Berardinelli-Seip congenital lipodystrophy 2/SEIPIN determines brown adipose tissue maintenance and thermogenic programing.Mol Metab. 2020 Jun;36:100971. doi: 10.1016/j.molmet.2020.02.014. Epub 2020 Mar 4. Mol Metab. 2020. PMID: 32246911 Free PMC article.
-
Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity.Biochimie. 2017 Mar;134:62-70. doi: 10.1016/j.biochi.2016.09.007. Epub 2016 Sep 10. Biochimie. 2017. PMID: 27621146 Review.
-
UCP1-independent thermogenesis.Biochem J. 2020 Feb 14;477(3):709-725. doi: 10.1042/BCJ20190463. Biochem J. 2020. PMID: 32059055 Review.
Cited by
-
Dose-Dependent Effect of Melatonin on BAT Thermogenesis in Zücker Diabetic Fatty Rat: Future Clinical Implications for Obesity.Antioxidants (Basel). 2022 Aug 25;11(9):1646. doi: 10.3390/antiox11091646. Antioxidants (Basel). 2022. PMID: 36139720 Free PMC article.
-
Pharmacological Action of a Pregnane Glycoside, Russelioside B, in Dietary Obese Rats: Impact on Weight Gain and Energy Expenditure.Front Pharmacol. 2018 Aug 30;9:990. doi: 10.3389/fphar.2018.00990. eCollection 2018. Front Pharmacol. 2018. PMID: 30214412 Free PMC article.
-
Intracellular glycolysis in brown adipose tissue is essential for optogenetically induced nonshivering thermogenesis in mice.Sci Rep. 2018 Apr 27;8(1):6672. doi: 10.1038/s41598-018-25265-3. Sci Rep. 2018. PMID: 29704006 Free PMC article.
-
Central vs. Peripheral Action of Thyroid Hormone in Adaptive Thermogenesis: A Burning Topic.Cells. 2021 May 27;10(6):1327. doi: 10.3390/cells10061327. Cells. 2021. PMID: 34071979 Free PMC article. Review.
-
A Novel Mix of Polyphenols and Micronutrients Reduces Adipogenesis and Promotes White Adipose Tissue Browning via UCP1 Expression and AMPK Activation.Cells. 2023 Feb 24;12(5):714. doi: 10.3390/cells12050714. Cells. 2023. PMID: 36899850 Free PMC article.
References
-
- Schuler A.M., Gower B.A., Matern D., Rinaldo P., Vockley J., Wood P.A. Synergistic heterozygosity in mice with inherited enzyme deficiencies of mitochondrial fatty acid beta-oxidation. Molecular Genetics and Metabolism. 2005;85(1):7–11. - PubMed
-
- Hankir M.K. Dissociation between brown adipose tissue 18F-FDG uptake and thermogenesis in uncoupling protein 1-deficient mice. Journal of Nuclear Medicine. 2017;58(7):1100–1103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

