Epithelial-mesenchymal crosstalk influences cellular behavior in a 3D alveolus-fibroblast model system
- PMID: 29175081
- PMCID: PMC5748390
- DOI: 10.1016/j.biomaterials.2017.11.008
Epithelial-mesenchymal crosstalk influences cellular behavior in a 3D alveolus-fibroblast model system
Abstract
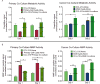
Interactions between lung epithelium and interstitial fibroblasts are increasingly recognized as playing a major role in the progression of several lung pathologies, including cancer. Three-dimensional in vitro co-culture systems offer tissue-relevant platforms to study the signaling interplay between diseased and healthy cell types. Such systems provide a controlled environment in which to probe the mechanisms involved in epithelial-mesenchymal crosstalk. To recapitulate the native alveolar tissue architecture, we employed a cyst templating technique to culture alveolar epithelial cells on photodegradable microspheres and subsequently encapsulated the cell-laden spheres within poly (ethylene glycol) (PEG) hydrogels containing dispersed pulmonary fibroblasts. A fibroblast cell line (CCL-210) was co-cultured with either healthy mouse alveolar epithelial primary cells or a cancerous alveolar epithelial cell line (A549) to probe the influence of tumor-stromal interactions on proliferation, migration, and matrix remodeling. In 3D co-culture, cancerous epithelial cells and fibroblasts had higher proliferation rates. When examining fibroblast motility, the fibroblasts migrated faster when co-cultured with cancerous A549 cells. Finally, a fluorescent peptide reporter for matrix metalloproteinase (MMP) activity revealed increased MMP activity when A549s and fibroblasts were co-cultured. When MMP activity was inhibited or when cells were cultured in gels with a non-degradable crosslinker, fibroblast migration was dramatically suppressed, and the increase in cancer cell proliferation in co-culture was abrogated. Together, this evidence supports the idea that there is an exchange between the alveolar epithelium and surrounding fibroblasts during cancer progression that depends on MMP activity and points to potential signaling routes that merit further investigation to determine targets for cancer treatment.
Keywords: Alveolar epithelium; Cancer; Co-culture; Fibroblast; Hydrogel; Matrix metalloproteinases.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
In vitro model alveoli from photodegradable microsphere templates.Biomater Sci. 2015 Jun;3(6):821-32. doi: 10.1039/c5bm00034c. Epub 2015 Mar 27. Biomater Sci. 2015. PMID: 26221842 Free PMC article.
-
Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics.Acta Biomater. 2017 Mar 1;50:437-449. doi: 10.1016/j.actbio.2016.12.049. Epub 2016 Dec 27. Acta Biomater. 2017. PMID: 28039063 Free PMC article.
-
The effect of matrix characteristics on fibroblast proliferation in 3D gels.Biomaterials. 2010 Nov;31(32):8454-64. doi: 10.1016/j.biomaterials.2010.07.046. Epub 2010 Aug 3. Biomaterials. 2010. PMID: 20684983
-
Epithelial-mesenchymal crosstalk in COPD: An update from in vitro model studies.Int J Biochem Cell Biol. 2020 Aug;125:105775. doi: 10.1016/j.biocel.2020.105775. Epub 2020 May 28. Int J Biochem Cell Biol. 2020. PMID: 32473924 Review.
-
The alveolus: Our current knowledge of how the gas exchange unit of the lung is constructed and repaired.Curr Top Dev Biol. 2024;159:59-129. doi: 10.1016/bs.ctdb.2024.01.002. Epub 2024 Mar 6. Curr Top Dev Biol. 2024. PMID: 38729684 Review.
Cited by
-
Probing coordinated co-culture cancer related motility through differential micro-compartmentalized elastic substrates.Sci Rep. 2020 Oct 28;10(1):18519. doi: 10.1038/s41598-020-74575-y. Sci Rep. 2020. PMID: 33116169 Free PMC article.
-
Biomaterials to model and measure epithelial cancers.Nat Rev Mater. 2018 Nov;3(11):418-430. Epub 2018 Sep 6. Nat Rev Mater. 2018. PMID: 30416759 Free PMC article.
-
In Vitro 3D Cultures to Model the Tumor Microenvironment.Cancers (Basel). 2021 Jun 13;13(12):2970. doi: 10.3390/cancers13122970. Cancers (Basel). 2021. PMID: 34199324 Free PMC article. Review.
-
Biofabrication of phenotypic pulmonary fibrosis assays.Biofabrication. 2019 Jun 19;11(3):032005. doi: 10.1088/1758-5090/ab2286. Biofabrication. 2019. PMID: 31215521 Free PMC article. Review.
-
Tissue-informed engineering strategies for modeling human pulmonary diseases.Am J Physiol Lung Cell Mol Physiol. 2019 Feb 1;316(2):L303-L320. doi: 10.1152/ajplung.00353.2018. Epub 2018 Nov 21. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30461289 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

