Peripheral vascular atherosclerosis in a novel PCSK9 gain-of-function mutant Ossabaw miniature pig model
- PMID: 29175268
- PMCID: PMC5811343
- DOI: 10.1016/j.trsl.2017.10.007
Peripheral vascular atherosclerosis in a novel PCSK9 gain-of-function mutant Ossabaw miniature pig model
Abstract
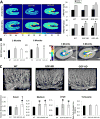
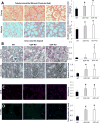
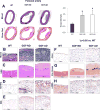
Hypercholesterolemia is a major risk factor for atherosclerosis. Remaining challenges in the management of atherosclerosis necessitate development of animal models that mimic human pathophysiology. We characterized a novel mutant pig model with DNA transposition of D374Y gain-of-function (GOF) cDNA of chimp proprotein convertase subtilisin/kexin type-9 (PCSK9), and tested the hypothesis that it would develop peripheral vascular remodeling and target organ injury in the kidney. Wild-type or PCSK9-GOF Ossabaw miniature pigs fed a standard or atherogenic diet (AD) (n = 7 each) were studied in vivo after 3 and 6 months of diet. Single-kidney hemodynamics and function were studied using multidetector computed tomography and kidney oxygenation by blood oxygen level-dependent magnetic resonance imaging. The renal artery was evaluated by intravascular ultrasound, aortic stiffness by multidetector computed tomography, and kidney stiffness by magnetic resonance elastography. Subsequent ex vivo studies included the renal artery endothelial function and morphology of abdominal aorta, renal, and femoral arteries by histology. Compared with wild type, PCSK9-GOF pigs had elevated cholesterol, triglyceride, and blood pressure levels at 3 and 6 months. Kidney stiffness increased in GOF groups, but aortic stiffness only in GOF-AD. Hypoxia, intrarenal fat deposition, oxidative stress, and fibrosis were observed in both GOF groups, whereas kidney function remained unchanged. Peripheral arteries in GOF groups showed medial thickening and development of atheromatous plaques. Renal endothelial function was impaired only in GOF-AD. Therefore, the PCSK9-GOF mutation induces rapid development of atherosclerosis in peripheral vessels of Ossabaw pigs, which is exacerbated by a high-cholesterol diet. This model may be useful for preclinical studies of atherosclerosis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have read the journal’s policy on disclosure of potential conflicts of interest.
Figures


References
-
- Celermajer DS, Chow CK, Marijon E, Anstey NM, Woo KS. Cardiovascular disease in the developing world: prevalences, patterns, and the potential of early disease detection. J Am Coll Cardiol. 2012;60:1207–16. - PubMed
-
- Sillesen H, Falk E. Why not screen for subclinical atherosclerosis? Lancet. 2011;378:645–6. - PubMed
-
- Breslow JL. Mouse models of atherosclerosis. Science. 1996;272:685–8. - PubMed
-
- Mohler ER, 3rd, Sarov-Blat L, Shi Y, Hamamdzic D, Zalewski A, Macphee C, et al. Site-specific atherogenic gene expression correlates with subsequent variable lesion development in coronary and peripheral vasculature. Arterioscler Thromb Vasc Biol. 2008;28:850–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

