Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade
- PMID: 29175753
- PMCID: PMC5716955
- DOI: 10.1016/j.redox.2017.11.012
Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade
Abstract
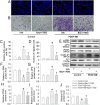
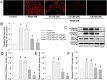
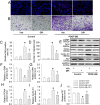
Phenotypic switch of vascular smooth muscle cells (VSMCs) is characterized by increased expressions of VSMC synthetic markers and decreased levels of VSMC contractile markers, which is an important step for VSMC proliferation and migration during the development and progression of cardiovascular diseases including atherosclerosis. Chicoric acid (CA) is identified to exert powerful cardiovascular protective effects. However, little is known about the effects of CA on VSMC biology. Herein, in cultured VSMCs, we showed that pretreatment with CA dose-dependently suppressed platelet-derived growth factor type BB (PDGF-BB)-induced VSMC phenotypic alteration, proliferation and migration. Mechanistically, PDGF-BB-treated VSMCs exhibited higher mammalian target of rapamycin (mTOR) and P70S6K phosphorylation, which was attenuated by CA pretreatment, diphenyleneiodonium chloride (DPI), reactive oxygen species (ROS) scavenger N-acetyl-l-cysteine (NAC) and nuclear factor-κB (NFκB) inhibitor Bay117082. PDGF-BB-triggered ROS production and p65-NFκB activation were inhibited by CA. In addition, both NAC and DPI abolished PDGF-BB-evoked p65-NFκB nuclear translocation, phosphorylation and degradation of Inhibitor κBα (IκBα). Of note, blockade of ROS/NFκB/mTOR/P70S6K signaling cascade prevented PDGF-BB-evoked VSMC phenotypic transformation, proliferation and migration. CA treatment prevented intimal hyperplasia and vascular remodeling in rat models of carotid artery ligation in vivo. These results suggest that CA impedes PDGF-BB-induced VSMC phenotypic switching, proliferation, migration and neointima formation via inhibition of ROS/NFκB/mTOR/P70S6K signaling cascade.
Keywords: Chicoric acid; Migration; PDGF-BB; Proliferation; VSMCs.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





References
-
- Cao T., Zhang L., Yao L.L., Zheng F., Wang L., Yang J.Y., Guo L.Y., Li X.Y., Yan Y.W., Pan Y.M., Jiang M., Chen L., Tang J.M., Chen S.Y., Jia N. S100B promotes injury-induced vascular remodeling through modulating smooth muscle phenotype. Biochim. Biophys. Acta. 2017 - PubMed
-
- Zhu L.H., Huang L., Zhang X., Zhang P., Zhang S.M., Guan H., Zhang Y., Zhu X.Y., Tian S., Deng K., Li H. Mindin regulates vascular smooth muscle cell phenotype and prevents neointima formation. Clin. Sci. 2015;129:129–145. - PubMed
-
- Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. Vascular smooth muscle cell in atherosclerosis. Acta Physiol. 2015;214:33–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

