Avian Influenza H5N6 Viruses Exhibit Differing Pathogenicities and Transmissibilities in Mammals
- PMID: 29176564
- PMCID: PMC5701206
- DOI: 10.1038/s41598-017-16139-1
Avian Influenza H5N6 Viruses Exhibit Differing Pathogenicities and Transmissibilities in Mammals
Erratum in
-
Author Correction: Avian Influenza H5N6 Viruses Exhibit Differing Pathogenicities and Transmissibilities in Mammals.Sci Rep. 2018 Jun 8;8(1):9084. doi: 10.1038/s41598-018-26658-0. Sci Rep. 2018. PMID: 29884792 Free PMC article.
Abstract
Since 2013, highly pathogenic avian influenza H5N6 viruses have emerged in poultry and caused sporadic infections in humans, increasing global concerns regarding their potential as human pandemic threats. Here, we characterized the receptor-binding specificities, pathogenicities and transmissibilities of three H5N6 viruses isolated from poultry in China. The surface genes hemagglutinin (HA) and neuraminidase (NA) were closely related to the human-originating strain A/Changsha/1/2014 (H5N6). Phylogenetic analyses showed that the HA genes were clustered in the 2.3.4.4 clade, and the NA genes were derived from H6N6 viruses. These H5N6 viruses bound both α-2,3-linked and α-2,6-linked sialic acid receptors, but they exhibited different pathogenicities in mice. In addition, one virus was fully infective and transmissible by direct contact in guinea pigs. These results highlight the importance of monitoring the continual adaptation of H5N6 viruses in poultry due to their potential threat to human health.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

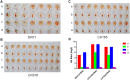



Similar articles
-
Highly Pathogenic Avian Influenza H5N6 Viruses Exhibit Enhanced Affinity for Human Type Sialic Acid Receptor and In-Contact Transmission in Model Ferrets.J Virol. 2016 Jun 24;90(14):6235-6243. doi: 10.1128/JVI.00127-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27122581 Free PMC article.
-
A study of the relationship between human infection with avian influenza a (H5N6) and environmental avian influenza viruses in Fujian, China.BMC Infect Dis. 2019 Sep 2;19(1):762. doi: 10.1186/s12879-019-4145-6. BMC Infect Dis. 2019. PMID: 31477028 Free PMC article.
-
Molecular Evolution and Emergence of H5N6 Avian Influenza Virus in Central China.J Virol. 2017 May 26;91(12):e00143-17. doi: 10.1128/JVI.00143-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28404845 Free PMC article.
-
Emergence and dissemination of clade 2.3.4.4 H5Nx influenza viruses-how is the Asian HPAI H5 lineage maintained.Curr Opin Virol. 2016 Feb;16:158-163. doi: 10.1016/j.coviro.2016.02.005. Epub 2016 Mar 15. Curr Opin Virol. 2016. PMID: 26991931 Review.
-
[Novel function of plasminogen-binding activity of the NA determines the pathogenicity of influenza A virus].Uirusu. 2004 Jun;54(1):83-91. doi: 10.2222/jsv.54.83. Uirusu. 2004. PMID: 15449908 Review. Japanese.
Cited by
-
Adaptive amino acid substitutions enable transmission of an H9N2 avian influenza virus in guinea pigs.Sci Rep. 2019 Dec 24;9(1):19734. doi: 10.1038/s41598-019-56122-6. Sci Rep. 2019. PMID: 31875046 Free PMC article.
-
Risk of Environmental Exposure to H7N9 Influenza Virus via Airborne and Surface Routes in a Live Poultry Market in Hebei, China.Front Cell Infect Microbiol. 2021 Jun 7;11:688007. doi: 10.3389/fcimb.2021.688007. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34164347 Free PMC article.
-
Patient-derived avian influenza A (H5N6) virus is highly pathogenic in mice but can be effectively treated by anti-influenza polyclonal antibodies.Emerg Microbes Infect. 2018 Jun 13;7(1):107. doi: 10.1038/s41426-018-0113-2. Emerg Microbes Infect. 2018. PMID: 29899428 Free PMC article.
-
A review of H5Nx avian influenza viruses.Ther Adv Vaccines Immunother. 2019 Feb 22;7:2515135518821625. doi: 10.1177/2515135518821625. eCollection 2019. Ther Adv Vaccines Immunother. 2019. PMID: 30834359 Free PMC article. Review.
-
Divergent Pathogenesis and Transmission of Highly Pathogenic Avian Influenza A(H5N1) in Swine.Emerg Infect Dis. 2024 Apr;30(4):738-751. doi: 10.3201/eid3004.231141. Epub 2024 Mar 13. Emerg Infect Dis. 2024. PMID: 38478379 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

