A permanent window for the murine lung enables high-resolution imaging of cancer metastasis
- PMID: 29176592
- PMCID: PMC5755704
- DOI: 10.1038/nmeth.4511
A permanent window for the murine lung enables high-resolution imaging of cancer metastasis
Abstract
Stable, high-resolution intravital imaging of the lung has become possible through the utilization of vacuum-stabilized imaging windows. However, this technique is extremely invasive and limited to only hours in duration. Here we describe a minimally invasive, permanently implantable window for high-resolution intravital imaging of the murine lung that allows the mouse to survive surgery, recover from anesthesia, and breathe independently. Compared to vacuum-stabilized windows, this window produces the same high-quality images without vacuum-induced artifacts; it is also less invasive, which allows imaging of the same lung tissue over a period of weeks. We further adapt the technique of microcartography for reliable relocalization of the same cells longitudinally. Using commonly employed experimental, as well as more clinically relevant, spontaneous metastasis models, we visualize all stages of metastatic seeding, including: tumor cell arrival; extravasation; growth and progression to micrometastases; as well as tumor microenvironment of metastasis function, the hallmark of hematogenous dissemination of tumor cells.
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper.
Figures
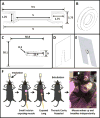

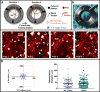

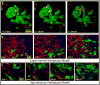
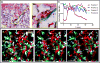
Comment in
-
A new window sheds light on lung tumor metastasis.Lab Anim (NY). 2018 Jan 31;47(2):26. doi: 10.1038/laban.1409. Lab Anim (NY). 2018. PMID: 29384520 No abstract available.
References
-
- Martin EA. Concise Medical Dictionary. Oxford University Press; 2010.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

