Dyslipidaemia in nephrotic syndrome: mechanisms and treatment
- PMID: 29176657
- PMCID: PMC5770189
- DOI: 10.1038/nrneph.2017.155
Dyslipidaemia in nephrotic syndrome: mechanisms and treatment
Erratum in
-
Dyslipidaemia in nephrotic syndrome: mechanisms and treatment.Nat Rev Nephrol. 2017 Dec 13;14(1):70. doi: 10.1038/nrneph.2017.175. Nat Rev Nephrol. 2017. PMID: 29234164
Abstract
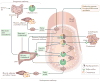
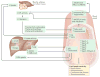
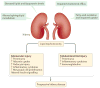
Nephrotic syndrome is a highly prevalent disease that is associated with high morbidity despite notable advances in its treatment. Many of the complications of nephrotic syndrome, including the increased risk of atherosclerosis and thromboembolism, can be linked to dysregulated lipid metabolism and dyslipidaemia. These abnormalities include elevated plasma levels of cholesterol, triglycerides and the apolipoprotein B-containing lipoproteins VLDL and IDL; decreased lipoprotein lipase activity in the endothelium, muscle and adipose tissues; decreased hepatic lipase activity; and increased levels of the enzyme PCSK9. In addition, there is an increase in the plasma levels of immature HDL particles and reduced cholesterol efflux. Studies from the past few years have markedly improved our understanding of the molecular pathogenesis of nephrotic syndrome-associated dyslipidaemia, and also heightened our awareness of the associated exacerbated risks of cardiovascular complications, progressive kidney disease and thromboembolism. Despite the absence of clear guidelines regarding treatment, various strategies are being increasingly utilized, including statins, bile acid sequestrants, fibrates, nicotinic acid and ezetimibe, as well as lipid apheresis, which seem to also induce partial or complete clinical remission of nephrotic syndrome in a substantial percentage of patients. Future potential treatments will likely also include inhibition of PCSK9 using recently-developed anti-PCSK9 monoclonal antibodies and small inhibitory RNAs, as well as targeting newly identified molecular regulators of lipid metabolism that are dysregulated in nephrotic syndrome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Greenbaum LA, Benndorf R, Smoyer WE. Childhood nephrotic syndrome — current and future therapies. Nat Rev Nephrol. 2012;8:445–458. - PubMed
-
- Clark AG, Barratt TM. In: Pediatric Nephrology. Barratt TM, Avner ED, Harmon WE, editors. Lippincott Williams & Wilkins; 1998. pp. 731–747.
-
- McEnery PT, Strife CF. Nephrotic syndrome in childhood. Management and treatment in patients with minimal change disease, mesangial proliferation, or focal glomerulosclerosis. Pediatr Clin North Am. 1982;89:875–894. - PubMed
-
- Nash MA, Edelmann CMJ, Bernstein J, Barnett HL. In: Pediatric Kidney Disease. Edelmann CMJ, editor. Little; 1992. pp. 1247–1266.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

