Pseudomonas aeruginosa exoproducts determine antibiotic efficacy against Staphylococcus aureus
- PMID: 29176757
- PMCID: PMC5720819
- DOI: 10.1371/journal.pbio.2003981
Pseudomonas aeruginosa exoproducts determine antibiotic efficacy against Staphylococcus aureus
Abstract
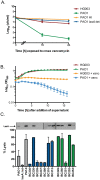
Chronic coinfections of Staphylococcus aureus and Pseudomonas aeruginosa frequently fail to respond to antibiotic treatment, leading to significant patient morbidity and mortality. Currently, the impact of interspecies interaction on S. aureus antibiotic susceptibility remains poorly understood. In this study, we utilize a panel of P. aeruginosa burn wound and cystic fibrosis (CF) lung isolates to demonstrate that P. aeruginosa alters S. aureus susceptibility to bactericidal antibiotics in a variable, strain-dependent manner and further identify 3 independent interactions responsible for antagonizing or potentiating antibiotic activity against S. aureus. We find that P. aeruginosa LasA endopeptidase potentiates lysis of S. aureus by vancomycin, rhamnolipids facilitate proton-motive force-independent tobramycin uptake, and 2-heptyl-4-hydroxyquinoline N-oxide (HQNO) induces multidrug tolerance in S. aureus through respiratory inhibition and reduction of cellular ATP. We find that the production of each of these factors varies between clinical isolates and corresponds to the capacity of each isolate to alter S. aureus antibiotic susceptibility. Furthermore, we demonstrate that vancomycin treatment of a S. aureus mouse burn infection is potentiated by the presence of a LasA-producing P. aeruginosa population. These findings demonstrate that antibiotic susceptibility is complex and dependent not only upon the genotype of the pathogen being targeted, but also on interactions with other microorganisms in the infection environment. Consideration of these interactions will improve the treatment of polymicrobial infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Pseudomonas aeruginosa Alters Staphylococcus aureus Sensitivity to Vancomycin in a Biofilm Model of Cystic Fibrosis Infection.mBio. 2017 Jul 18;8(4):e00873-17. doi: 10.1128/mBio.00873-17. mBio. 2017. PMID: 28720732 Free PMC article.
-
Pseudomonas aeruginosa PA14 Enhances the Efficacy of Norfloxacin against Staphylococcus aureus Newman Biofilms.J Bacteriol. 2020 Aug 25;202(18):e00159-20. doi: 10.1128/JB.00159-20. Print 2020 Aug 25. J Bacteriol. 2020. PMID: 32661077 Free PMC article.
-
A Pseudomonas aeruginosa Antimicrobial Affects the Biogeography but Not Fitness of Staphylococcus aureus during Coculture.mBio. 2021 Mar 30;12(2):e00047-21. doi: 10.1128/mBio.00047-21. mBio. 2021. PMID: 33785630 Free PMC article.
-
Friends or enemies? The complicated relationship between Pseudomonas aeruginosa and Staphylococcus aureus.Mol Microbiol. 2021 Jul;116(1):1-15. doi: 10.1111/mmi.14699. Epub 2021 Mar 8. Mol Microbiol. 2021. PMID: 33576132 Review.
-
Help, hinder, hide and harm: what can we learn from the interactions between Pseudomonas aeruginosa and Staphylococcus aureus during respiratory infections?Thorax. 2019 Jul;74(7):684-692. doi: 10.1136/thoraxjnl-2018-212616. Epub 2019 Feb 18. Thorax. 2019. PMID: 30777898 Free PMC article. Review.
Cited by
-
The inhibitory effects of Staphylococcus aureus on the antibiotic susceptibility and virulence factors of Pseudomonas aeruginosa: A549 cell line model.AMB Express. 2021 Mar 30;11(1):50. doi: 10.1186/s13568-021-01210-y. AMB Express. 2021. PMID: 33786713 Free PMC article.
-
Enterococcus faecalis thrives in dual-species biofilm models under iron-rich conditions.Arch Microbiol. 2022 Nov 16;204(12):710. doi: 10.1007/s00203-022-03309-7. Arch Microbiol. 2022. PMID: 36383258
-
High Occurrence of Bacterial Competition Among Clinically Documented Opportunistic Pathogens Including Achromobacter xylosoxidans in Cystic Fibrosis.Front Microbiol. 2020 Sep 10;11:558160. doi: 10.3389/fmicb.2020.558160. eCollection 2020. Front Microbiol. 2020. PMID: 33013789 Free PMC article.
-
Mutation of Pseudomonas aeruginosa lasI/rhlI diminishes its cytotoxicity, oxidative stress, inflammation, and apoptosis on THP-1 macrophages.Microbiol Spectr. 2024 Oct 3;12(10):e0414623. doi: 10.1128/spectrum.04146-23. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162513 Free PMC article.
-
Staphylococcus aureus in Polymicrobial Skinand Soft Tissue Infections: Impact of Inter-Species Interactionsin Disease Outcome.Antibiotics (Basel). 2023 Jul 8;12(7):1164. doi: 10.3390/antibiotics12071164. Antibiotics (Basel). 2023. PMID: 37508260 Free PMC article. Review.
References
-
- Conlon BP. Staphylococcus aureus chronic and relapsing infections: Evidence of a role for persister cells: An investigation of persister cells, their formation and their role in S. aureus disease. Bioessays. 2014;36(10):991–6. Epub 2014/08/08. doi: 10.1002/bies.201400080 - DOI - PubMed
-
- Levin BR, Rozen DE. Non-inherited antibiotic resistance. Nat Rev Microbiol. 2006;4(7):556–62. doi: 10.1038/nrmicro1445 - DOI - PubMed
-
- Kubicek-Sutherland JZ, Lofton H, Vestergaard M, Hjort K, Ingmer H, Andersson DI. Antimicrobial peptide exposure selects for Staphylococcus aureus resistance to human defence peptides. J Antimicrob Chemother. 2017;72(1):115–27. doi: 10.1093/jac/dkw381 - DOI - PMC - PubMed
-
- Kong EF, Tsui C, Kucharikova S, Andes D, Van Dijck P, Jabra-Rizk MA. Commensal Protection of Staphylococcus aureus against Antimicrobials by Candida albicans Biofilm Matrix. MBio. 2016;7(5). doi: 10.1128/mBio.01365-16 - DOI - PMC - PubMed
-
- Hoffman LR, Deziel E, D’Argenio DA, Lepine F, Emerson J, McNamara S, et al. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2006;103(52):19890–5. doi: 10.1073/pnas.0606756104 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

