Vaccine-elicited memory CD4+ T cell expansion is impaired in the lungs during tuberculosis
- PMID: 29176787
- PMCID: PMC5720822
- DOI: 10.1371/journal.ppat.1006704
Vaccine-elicited memory CD4+ T cell expansion is impaired in the lungs during tuberculosis
Abstract
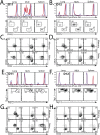
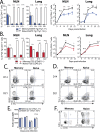
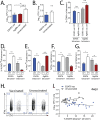
Immunological memory is the key biological process that makes vaccines possible. Although tuberculosis vaccines elicit protective immunity in animals, few provide durable protection. To understand why protection is transient, we evaluated the ability of memory CD4+ T cells to expand, differentiate, and control Mycobacterium tuberculosis. Both naïve and memory CD4+ T cells initially proliferated exponentially, and the accumulation of memory T cells in the lung correlated with early bacterial control. However, later during infection, memory CD4+ T cell proliferation was curtailed and no protection was observed. We show that memory CD4+ T cells are first activated in the LN and their recruitment to the lung attenuates bacterial growth. However, their interaction with Mtb-infected macrophages does not promote continued proliferation. We conclude that a lack of sustained expansion by memory-derived T cells in the lung limits the durability of their protection, linking their slower expansion with transient protection in vaccinated mice.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PEM, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. CLIN INFECT DIS. 2014;58: 470–480. doi: 10.1093/cid/cit790 - DOI - PubMed
-
- Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381: 1021–1028. doi: 10.1016/S0140-6736(13)60177-4 - DOI - PMC - PubMed
-
- Millet J-P, Shaw E, Orcau À, Casals M, Miro JM, Caylà JA, et al. Tuberculosis recurrence after completion treatment in a European city: reinfection or relapse? Mokrousov I, editor. PLoS ONE. 2013;8: e64898 doi: 10.1371/journal.pone.0064898 - DOI - PMC - PubMed
-
- Jameson SC, Masopust D. Diversity in T Cell Memory: An Embarrassment of Riches. Immunity. Elsevier Inc; 2009;31: 859–871. doi: 10.1016/j.immuni.2009.11.007 - DOI - PMC - PubMed
-
- Kamath AB, Behar SM. Anamnestic responses of mice following Mycobacterium tuberculosis infection. Infect Immun. 2005;73: 6110–6118. doi: 10.1128/IAI.73.9.6110-6118.2005 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

