Neuroprotection and neuroregeneration of retinal ganglion cells after intravitreal carbon monoxide release
- PMID: 29176876
- PMCID: PMC5703485
- DOI: 10.1371/journal.pone.0188444
Neuroprotection and neuroregeneration of retinal ganglion cells after intravitreal carbon monoxide release
Abstract
Purpose: Retinal ischemia induces apoptosis leading to neurodegeneration and vision impairment. Carbon monoxide (CO) in gaseous form showed cell-protective and anti-inflammatory effects after retinal ischemia-reperfusion-injury (IRI). These effects were also demonstrated for the intravenously administered CO-releasing molecule (CORM) ALF-186. This article summarizes the results of intravitreally released CO to assess its suitability as a neuroprotective and neuroregenerative agent.
Methods: Water-soluble CORM ALF-186 (25 μg), PBS, or inactivated ALF (iALF) (all 5 μl) were intravitreally applied into the left eyes of rats directly after retinal IRI for 1 h. Their right eyes remained unaffected and were used for comparison. Retinal tissue was harvested 24 h after intervention to analyze mRNA or protein expression of Caspase-3, pERK1/2, p38, HSP70/90, NF-kappaB, AIF-1 (allograft inflammatory factor), TNF-α, and GAP-43. Densities of fluorogold-prelabeled retinal ganglion cells (RGC) were examined in flat-mounted retinae seven days after IRI and were expressed as mean/mm2. The ability of RGC to regenerate their axon was evaluated two and seven days after IRI using retinal explants in laminin-1-coated cultures. Immunohistochemistry was used to analyze the different cell types growing out of the retinal explants.
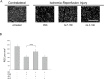
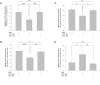
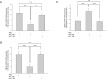
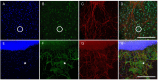
Results: Compared to the RGC-density in the contralateral right eyes (2804±214 RGC/mm2; data are mean±SD), IRI+PBS injection resulted in a remarkable loss of RGC (1554±159 RGC/mm2), p<0.001. Intravitreally injected ALF-186 immediately after IRI provided RGC protection and reduced the extent of RGC-damage (IRI+PBS 1554±159 vs. IRI+ALF 2179±286, p<0.001). ALF-186 increased the IRI-mediated phosphorylation of MAP-kinase p38. Anti-apoptotic and anti-inflammatory effects were detectable as Caspase-3, NF-kappaB, TNF-α, and AIF-1 expression were significantly reduced after IRI+ALF in comparison to IRI+PBS or IRI+iALF. Gap-43 expression was significantly increased after IRI+ALF. iALF showed effects similar to PBS. The intrinsic regenerative potential of RGC-axons was induced to nearly identical levels after IRI and ALF or iALF-treatment under growth-permissive conditions, although RGC viability differed significantly in both groups. Intravitreal CO further increased the IRI-induced migration of GFAP-positive cells out of retinal explants and their transdifferentiation, which was detected by re-expression of beta-III tubulin and nestin.
Conclusion: Intravitreal CORM ALF-186 protected RGC after IRI and stimulated their axons to regenerate in vitro. ALF conveyed anti-apoptotic, anti-inflammatory, and growth-associated signaling after IRI. CO's role in neuroregeneration and its effect on retinal glial cells needs further investigation.
Conflict of interest statement
Figures




Similar articles
-
The Carbon monoxide releasing molecule ALF-186 mediates anti-inflammatory and neuroprotective effects via the soluble guanylate cyclase ß1 in rats' retinal ganglion cells after ischemia and reperfusion injury.J Neuroinflammation. 2017 Jun 27;14(1):130. doi: 10.1186/s12974-017-0905-7. J Neuroinflammation. 2017. PMID: 28655348 Free PMC article.
-
The CORM ALF-186 Mediates Anti-Apoptotic Signaling via an Activation of the p38 MAPK after Ischemia and Reperfusion Injury in Retinal Ganglion Cells.PLoS One. 2016 Oct 20;11(10):e0165182. doi: 10.1371/journal.pone.0165182. eCollection 2016. PLoS One. 2016. PMID: 27764224 Free PMC article.
-
Postconditioning with inhaled carbon monoxide counteracts apoptosis and neuroinflammation in the ischemic rat retina.PLoS One. 2012;7(9):e46479. doi: 10.1371/journal.pone.0046479. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23029526 Free PMC article.
-
TNF-alpha signaling in glaucomatous neurodegeneration.Prog Brain Res. 2008;173:409-21. doi: 10.1016/S0079-6123(08)01128-X. Prog Brain Res. 2008. PMID: 18929124 Free PMC article. Review.
-
Carbon Monoxide Signaling: Examining Its Engagement with Various Molecular Targets in the Context of Binding Affinity, Concentration, and Biologic Response.Pharmacol Rev. 2022 Jul;74(3):823-873. doi: 10.1124/pharmrev.121.000564. Pharmacol Rev. 2022. PMID: 35738683 Free PMC article. Review.
Cited by
-
Medical Gas Therapy for Tissue, Organ, and CNS Protection: A Systematic Review of Effects, Mechanisms, and Challenges.Adv Sci (Weinh). 2022 May;9(13):e2104136. doi: 10.1002/advs.202104136. Epub 2022 Mar 4. Adv Sci (Weinh). 2022. PMID: 35243825 Free PMC article.
-
Carbon monoxide in intensive care medicine-time to start the therapeutic application?!Intensive Care Med Exp. 2020 Jan 9;8(1):2. doi: 10.1186/s40635-020-0292-8. Intensive Care Med Exp. 2020. PMID: 31919605 Free PMC article. Review.
-
Dysregulated Tear Film Proteins in Macular Edema Due to the Neovascular Age-Related Macular Degeneration Are Involved in the Regulation of Protein Clearance, Inflammation, and Neovascularization.J Clin Med. 2021 Jul 10;10(14):3060. doi: 10.3390/jcm10143060. J Clin Med. 2021. PMID: 34300228 Free PMC article.
-
Regenerative Potential of Carbon Monoxide in Adult Neural Circuits of the Central Nervous System.Int J Mol Sci. 2020 Mar 25;21(7):2273. doi: 10.3390/ijms21072273. Int J Mol Sci. 2020. PMID: 32218342 Free PMC article. Review.
-
The mito-QC Reporter for Quantitative Mitophagy Assessment in Primary Retinal Ganglion Cells and Experimental Glaucoma Models.Int J Mol Sci. 2020 Mar 10;21(5):1882. doi: 10.3390/ijms21051882. Int J Mol Sci. 2020. PMID: 32164182 Free PMC article.
References
-
- Prasad SS, Kojic L, Wen YH, Chen Z, Xiong W, Jia W, et al. Retinal gene expression after central retinal artery ligation: effects of ischemia and reperfusion. Invest Ophthalmol Vis Sci. 2010;51(12):6207–19. Epub 2010/08/13. doi: iovs.10-5632 [pii] doi: 10.1167/iovs.10-5632 . - DOI - PubMed
-
- Kim HS, Park CK. Retinal ganglion cell death is delayed by activation of retinal intrinsic cell survival program. Brain Res. 2005;1057(1–2):17–28. doi: 10.1016/j.brainres.2005.07.005 . - DOI - PubMed
-
- Hirsch S, Labes M, Bahr M. Changes in BDNF and neurotrophin receptor expression in degenerating and regenerating rat retinal ganglion cells. Restor Neurol Neurosci. 2000;17(2–3):125–34. . - PubMed
-
- Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005;1053:74–83. Epub 2005/09/24. doi: 1053/1/74 [pii] doi: 10.1196/annals.1344.007 . - DOI - PubMed
-
- Dijk F, Bergen AA, Kamphuis W. GAP-43 expression is upregulated in retinal ganglion cells after ischemia/reperfusion-induced damage. Exp Eye Res. 2007;84(5):858–67. Epub 2007/03/09. doi: S0014-4835(07)00027-9 [pii] doi: 10.1016/j.exer.2007.01.006 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

