The effect of Ca2+ ions and ionic strength on Mn(II) oxidation by spores of the marine Bacillus sp. SG-1
- PMID: 29176910
- PMCID: PMC5701786
- DOI: 10.1016/j.gca.2012.10.008
The effect of Ca2+ ions and ionic strength on Mn(II) oxidation by spores of the marine Bacillus sp. SG-1
Abstract
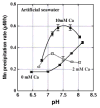
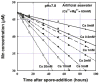
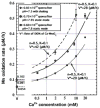
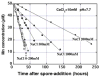
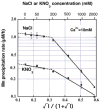
Manganese(IV) oxides, believed to form primarily through microbial activities, are extremely important mineral phases in marine environments where they scavenge a variety of trace elements and thereby control their distributions. The presence of various ions common in seawater are known to influence Mn oxide mineralogy yet little is known about the effect of these ions on the kinetics of bacterial Mn(II) oxidation and Mn oxide formation. We examined factors affecting bacterial Mn(II) oxidation by spores of the marine Bacillus sp. strain SG-1 in natural and artificial seawater of varying ionic conditions. Ca2+ concentration dramatically affected Mn(II) oxidation, while Mg2+, Sr2+, K+, Na+ and NO3- ions had no effect. The rate of Mn(II) oxidation at 10mM Ca2+ (seawater composition) was four or five times that without Ca2+. The relationship between Ca2+ content and oxidation rate demonstrates that the equilibrium constant is small (on the order of 0.1) and the binding coefficient is 0.5. The pH optimum for Mn(II) oxidation changed depending on the amount of Ca2+ present, suggesting that Ca2+ exerts a direct effect on the enzyme perhaps as a stabilizing bridge between polypeptide components. We also examined the effect of varying concentrations of NaCl or KNO3 (0 mM - 2000 mM) on the kinetics of Mn(II) oxidation in solutions containing 10 mM Ca2+. Mn(II) oxidation was unaffected by changes in ionic strength (I) below 0.2, but it was inhibited by increasing salt concentrations above this value. Our results suggest that the critical coagulation concentration is around 200 mM of salt (I = ca. 0.2), and that the ionic strength of seawater (I > 0.2) accelerates the precipitation of Mn oxides around the spores. Under these conditions, the aggregation of Mn oxides reduces the supply of dissolved O2 and/or Mn2+ and inhibits the Mn(II) -> Mn(III) step controlling the enzymatic oxidation of Mn(II). Our results suggest that the hardness and ionic strength of the aquatic environment at circumneutral pH strongly influences the rate of biologically mediated Mn(II) oxidation.
Figures

References
-
- Bailey EJ, Ollis FD. Biochemical Engneering Fundamentals. McGraw-Hill; 1986.
-
- Bargar JR, Tebo BM, Villinsk JE. In situ characterization of Mn(II) oxidation by spores of the marine Bacillus sp. strain SG-1. Geochim Cosmochim Acta. 2000;64:2737–2749.
-
- Bargar JR, Tebo BM, Bergmann U, Web SM, Glatzel P, Chiu V, Villalobos M. Biotic and Abioic Products of Mn(II) Oxidation by Spores of the Marine Bacillus sp. strain SG-1. Am Miner. 2005;90:143–154.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
