Cognitive Decline in Neuronal Aging and Alzheimer's Disease: Role of NMDA Receptors and Associated Proteins
- PMID: 29176942
- PMCID: PMC5687061
- DOI: 10.3389/fnins.2017.00626
Cognitive Decline in Neuronal Aging and Alzheimer's Disease: Role of NMDA Receptors and Associated Proteins
Abstract
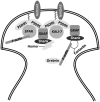
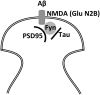
Molecular changes associated with neuronal aging lead to a decrease in cognitive capacity. Here we discuss these alterations at the level of brain regions, brain cells, and brain membrane and cytoskeletal proteins with an special focus in NMDA molecular changes through aging and its effect in cognitive decline and Alzheimer disease. Here, we propose that some neurodegenerative disorders, like Alzheimer's disease (AD), are characterized by an increase and acceleration of some of these changes.
Keywords: cognition; dendritic spines; neurotransmitter agents; tau proteins; therapies.
Figures


References
-
- Akashi K., Kakizaki T., Kamiya H., Fukaya M., Yamasaki M., Abe M., et al. (2009). NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, post-synaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J. Neurosci. 29, 10869–10882. 10.1523/JNEUROSCI.5531-08.2009 - DOI - PMC - PubMed
-
- Ardestani P. M., Evans A. K., Yi B., Nguyen T., Coutellier L., Shamloo M. (2017). Modulation of neuroinflammation and pathology in the 5XFAD mouse model of Alzheimer's disease using a biased and selective beta-1 adrenergic receptor partial agonist. Neuropharmacology 116, 371–386. 10.1016/j.neuropharm.2017.01.010 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

