Sound-Evoked Biceps Myogenic Potentials Reflect Asymmetric Vestibular Drive to Spastic Muscles in Chronic Hemiparetic Stroke Survivors
- PMID: 29176945
- PMCID: PMC5686083
- DOI: 10.3389/fnhum.2017.00535
Sound-Evoked Biceps Myogenic Potentials Reflect Asymmetric Vestibular Drive to Spastic Muscles in Chronic Hemiparetic Stroke Survivors
Abstract
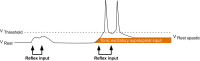
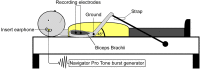
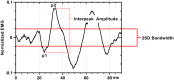
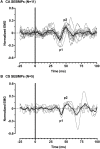
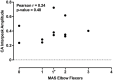
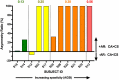
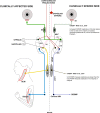
Aberrant vestibular nuclear function is proposed to be a principle driver of limb muscle spasticity after stroke. We sought to determine whether altered cortical modulation of descending vestibulospinal pathways post-stroke could impact the excitability of biceps brachii motoneurons. Twelve chronic hemispheric stroke survivors aged 46-68 years were enrolled. Sound evoked biceps myogenic potentials (SEBMPs) were recorded from the spastic and contralateral biceps muscles using surface EMG electrodes. We assessed the impact of descending vestibulospinal pathways on biceps muscle activity and evaluated the relationship between vestibular function and the severity of spasticity. Spastic SEBMP responses were recorded in 11/12 subjects. Almost 60% of stroke subjects showed evoked responses solely on the spastic side. These data strongly support the idea that vestibular drive is asymmetrically distributed to biceps motoneuron pools in hemiparetic spastic stroke survivors. This abnormal vestibular drive is very likely to be a factor mediating the striking differences in motoneuron excitability between the clinically affected and clinically spared sides. This study extends our previous observations on vestibular nuclear changes following hemispheric stroke and potentially sheds light on the underlying mechanisms of post-stroke spasticity.
Keywords: biceps brachii; motoneuron; spastic hypertonia; stroke; vestibular evoked myogenic potential; vestibular reflexes; vestibulospinal.
Figures
Similar articles
-
Ascending vestibular drive is asymmetrically distributed to the inferior oblique motoneuron pools in a subset of hemispheric stroke survivors.Clin Neurophysiol. 2016 Apr;127(4):2022-30. doi: 10.1016/j.clinph.2016.01.019. Epub 2016 Feb 18. Clin Neurophysiol. 2016. PMID: 26971485
-
Asymmetries in vestibular evoked myogenic potentials in chronic stroke survivors with spastic hypertonia: evidence for a vestibulospinal role.Clin Neurophysiol. 2014 Oct;125(10):2070-8. doi: 10.1016/j.clinph.2014.01.035. Epub 2014 Mar 12. Clin Neurophysiol. 2014. PMID: 24680197 Free PMC article.
-
Stretch reflex excitability in contralateral limbs of stroke survivors is higher than in matched controls.J Neuroeng Rehabil. 2019 Dec 5;16(1):154. doi: 10.1186/s12984-019-0623-8. J Neuroeng Rehabil. 2019. PMID: 31806032 Free PMC article.
-
The Contributions of Vestibular Evoked Myogenic Potentials and Acoustic Vestibular Stimulation to Our Understanding of the Vestibular System.Front Neurol. 2018 Jun 29;9:481. doi: 10.3389/fneur.2018.00481. eCollection 2018. Front Neurol. 2018. PMID: 30013504 Free PMC article. Review.
-
Vestibular evoked myogenic potentials in practice: Methods, pitfalls and clinical applications.Clin Neurophysiol Pract. 2019 Feb 26;4:47-68. doi: 10.1016/j.cnp.2019.01.005. eCollection 2019. Clin Neurophysiol Pract. 2019. PMID: 30949613 Free PMC article. Review.
Cited by
-
Ipsilateral motor pathways to the lower limb after stroke: Insights and opportunities.J Neurosci Res. 2021 Jun;99(6):1565-1578. doi: 10.1002/jnr.24822. Epub 2021 Mar 4. J Neurosci Res. 2021. PMID: 33665910 Free PMC article. Review.
References
-
- Bach L. M., Magoun H. W. (1947). The vestibular nuclei as an excitatory mechanism for the cord. J. Neurophysiol. 10, 331–337. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

