Reporter-Based Assays for High-Throughput Drug Screening against Mycobacterium abscessus
- PMID: 29176967
- PMCID: PMC5687050
- DOI: 10.3389/fmicb.2017.02204
Reporter-Based Assays for High-Throughput Drug Screening against Mycobacterium abscessus
Abstract
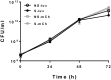
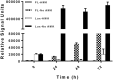
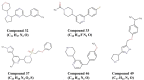
Mycobacterium abscessus is a non-tuberculous mycobacterium that causes pulmonary and non-pulmonary infections. M. abscessus is resistant to many chemotherapeutic agents and the current treatment options show poor clinical outcomes. Thus, there is a dire need to find new antimicrobials effective at killing M. abscessus. Screening drug libraries to identify potential antimicrobials has been impeded by the lack of validated HTS assays for M. abscessus. In this study, we developed two 384-well high-throughput screening assays using fluorescent and bioluminescent reporter strains of M. abscessus for drug discovery. Optimization of inoculum size, incubation time and the volume-per-well based on Z-factor and signal intensity yielded two complementary, robust tools for M. abscessus drug discovery with Z-factor > 0.8. The MIC of known drugs, amikacin and clarithromycin, as determined by bioluminescence was in agreement with the published MIC values. A proof-of-concept screen of 2,093 natural product-inspired compounds was conducted using the 384-well bioluminescent assay to identify novel scaffolds active against M. abscessus. Five active "hit" compounds identified in this pilot screen were confirmed and characterized by a CFU assay and MIC determination. Overall, we developed and validated a 384-well screen that offers simple, sensitive and fast screening of compounds for activity against this emerging pathogen. To our knowledge, this is the first reporter-based high-throughput screening study aimed at M. abscessus drug discovery.
Keywords: drug discovery and development; drug discovery screening; fluorescence; luminescence; non-tuberculous mycobacteria; reporter genes.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

