US6 Gene Deletion in Herpes Simplex Virus Type 2 Enhances Dendritic Cell Function and T Cell Activation
- PMID: 29176979
- PMCID: PMC5686121
- DOI: 10.3389/fimmu.2017.01523
US6 Gene Deletion in Herpes Simplex Virus Type 2 Enhances Dendritic Cell Function and T Cell Activation
Abstract
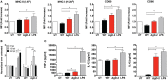
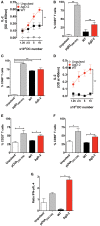
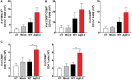
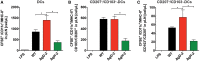
Herpes simplex virus (HSV) type 1 (HSV-1) and type 2 (HSV-2) produce lifelong infections that are associated with frequent asymptomatic or clinically apparent reactivation. Importantly, HSV express multiple virulence factors that negatively modulate innate and adaptive immune components. Notably, HSV interfere with dendritic cell (DC) viability and function, likely hindering the capacity of the host to mount effective immunity against these viruses. Recently, an HSV-2 virus that was deleted in glycoprotein D was engineered (designated ΔgD-2). The virus is propagated on a complementing cell line that expresses HSV-1 gD, which permits a single round of viral replication. ΔgD-2 is safe, immunogenic, and provided complete protection against vaginal or skin challenges with HSV-1 and HSV-2 in murine models. Here, we sought to assess the interaction of ΔgD-2 with DCs and found that, in contrast to wild-type (WT) virus which induces DC apoptosis, ΔgD-2 promoted their migration and capacity to activate naïve CD8+ and CD4+ T cells in vitro and in vivo. Furthermore, DCs exposed to the WT and ΔgD-2 virus experienced different unfolded protein responses. Mice primed with DCs infected with ΔgD-2 in vitro displayed significantly reduced infection and pathology after genital challenge with virulent HSV-2 compared to non-primed mice, suggesting that DCs play a role in the immune response to the vaccine strain.
Keywords: HSV type 2; adaptive immunity; apoptosis; dendritic cells; glycoprotein D; migration; unfolded protein response.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

