Patchy promiscuity: machine learning applied to predict the host specificity of Salmonella enterica and Escherichia coli
- PMID: 29177093
- PMCID: PMC5695212
- DOI: 10.1099/mgen.0.000135
Patchy promiscuity: machine learning applied to predict the host specificity of Salmonella enterica and Escherichia coli
Erratum in
-
Erratum: Patchy promiscuity: machine learning applied to predict the host specificity of Salmonella enterica and Escherichia coli.Microb Genom. 2018 Jun;4(6):e000193. doi: 10.1099/mgen.0.000193. Epub 2018 Jun 6. Microb Genom. 2018. PMID: 29874157 Free PMC article. No abstract available.
Abstract
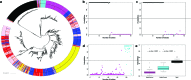
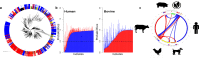
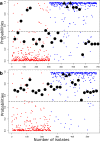
Salmonella enterica and Escherichia coli are bacterial species that colonize different animal hosts with sub-types that can cause life-threatening infections in humans. Source attribution of zoonoses is an important goal for infection control as is identification of isolates in reservoir hosts that represent a threat to human health. In this study, host specificity and zoonotic potential were predicted using machine learning in which Support Vector Machine (SVM) classifiers were built based on predicted proteins from whole genome sequences. Analysis of over 1000 S.enterica genomes allowed the correct prediction (67 -90 % accuracy) of the source host for S. Typhimurium isolates and the same classifier could then differentiate the source host for alternative serovars such as S. Dublin. A key finding from both phylogeny and SVM methods was that the majority of isolates were assigned to host-specific sub-clusters and had high host-specific SVM scores. Moreover, only a minor subset of isolates had high probability scores for multiple hosts, indicating generalists with genetic content that may facilitate transition between hosts. The same approach correctly identified human versus bovine E. coli isolates (83 % accuracy) and the potential of the classifier to predict a zoonotic threat was demonstrated using E. coli O157. This research indicates marked host restriction for both S. enterica and E. coli, with only limited isolate subsets exhibiting host promiscuity by gene content. Machine learning can be successfully applied to interrogate source attribution of bacterial isolates and has the capacity to predict zoonotic potential.
Keywords: E. coli; Salmonella; Support Vector Machine; host specificity; machine learning; zoonosis.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

