A physiological perspective on the origin and evolution of photosynthesis
- PMID: 29177446
- PMCID: PMC5972617
- DOI: 10.1093/femsre/fux056
A physiological perspective on the origin and evolution of photosynthesis
Abstract
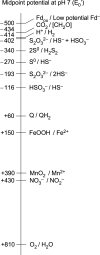
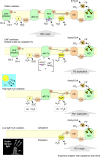
The origin and early evolution of photosynthesis are reviewed from an ecophysiological perspective. Earth's first ecosystems were chemotrophic, fueled by geological H2 at hydrothermal vents and, required flavin-based electron bifurcation to reduce ferredoxin for CO2 fixation. Chlorophyll-based phototrophy (chlorophototrophy) allowed autotrophs to generate reduced ferredoxin without electron bifurcation, providing them access to reductants other than H2. Because high-intensity, short-wavelength electromagnetic radiation at Earth's surface would have been damaging for the first chlorophyll (Chl)-containing cells, photosynthesis probably arose at hydrothermal vents under low-intensity, long-wavelength geothermal light. The first photochemically active pigments were possibly Zn-tetrapyrroles. We suggest that (i) after the evolution of red-absorbing Chl-like pigments, the first light-driven electron transport chains reduced ferredoxin via a type-1 reaction center (RC) progenitor with electrons from H2S; (ii) photothioautotrophy, first with one RC and then with two, was the bridge between H2-dependent chemolithoautotrophy and water-splitting photosynthesis; (iii) photothiotrophy sustained primary production in the photic zone of Archean oceans; (iv) photosynthesis arose in an anoxygenic cyanobacterial progenitor; (v) Chl a is the ancestral Chl; and (vi), anoxygenic chlorophototrophic lineages characterized so far acquired, by horizontal gene transfer, RCs and Chl biosynthesis with or without autotrophy, from the architects of chlorophototrophy-the cyanobacterial lineage.
Figures




References
-
- Albalat R, Cañestro C. Evolution by gene loss. Nat Rev Genet 2016;17:379–91. - PubMed
-
- Allen JF. A redox switch hypothesis for the origin of two light reactions in photosynthesis. FEBS Lett 2005;579:963–8. - PubMed
-
- Allen JF, Martin W. Evolutionary biology: out of thin air. Nature 2007;445:610–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

