Manipulation of Bryophyte Hosts by Pathogenic and Symbiotic Microbes
- PMID: 29177478
- PMCID: PMC6018959
- DOI: 10.1093/pcp/pcx182
Manipulation of Bryophyte Hosts by Pathogenic and Symbiotic Microbes
Erratum in
-
Erratum.Plant Cell Physiol. 2018 Apr 1;59(4):876. doi: 10.1093/pcp/pcy075. Plant Cell Physiol. 2018. PMID: 29718476 Free PMC article. No abstract available.
Abstract
The colonization of plant tissues by pathogenic and symbiotic microbes is associated with a strong and directed effort to reprogram host cells in order to permit, promote and sustain microbial growth. In response to colonization, hosts accommodate or sequester invading microbes by activating a set of complex regulatory programs that initiate symbioses or bolster defenses. Extensive research has elucidated a suite of molecular and physiological responses occurring in plant hosts and their microbial partners; however, this information is mostly limited to model systems representing evolutionarily young plant lineages such as angiosperms. The extent to which these processes are conserved across land plants is therefore poorly understood. In this review, we outline key aspects of host reprogramming that occur during plant-microbe interactions in early diverging land plants belonging to the bryophytes (liverworts, hornworts and mosses). We discuss how further knowledge of bryophyte-microbe interactions will advance our understanding of how plants and microbes co-operated and clashed during the conquest of land.
Figures

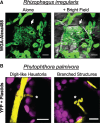
References
-
- Adams D.G., Duggan P.S. (2008) Cyanobacteria–bryophyte symbioses. J. Exp. Bot. 59: 1047–1058. - PubMed
-
- Balestrini R., Cosgrove D.J., Bonfante P. (2005) Differential location of alpha-expansin proteins during the accommodation of root cells to an arbuscular mycorrhizal fungus. Planta 220: 889–899. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

