Thrombopoietin receptor agonists for prevention and treatment of chemotherapy-induced thrombocytopenia in patients with solid tumours
- PMID: 29178132
- PMCID: PMC6486270
- DOI: 10.1002/14651858.CD012035.pub2
Thrombopoietin receptor agonists for prevention and treatment of chemotherapy-induced thrombocytopenia in patients with solid tumours
Abstract
Background: Chemotherapy-induced thrombocytopenia (CIT) is defined as a peripheral platelet count less than 100×109/L, with or without bleeding in cancer patients receiving myelosuppressive chemotherapy. CIT is a significant medical problem during chemotherapy, and it carries the risk of sub-optimal overall survival and bleeding. Alternative interventions to platelet transfusion are limited. Different stages of preclinical and clinical studies have examined the thrombopoietin receptor agonists (TPO-RAs) for CIT in patients with solid tumours.
Objectives: To assess the effects of TPO-RAs to prevent and treat CIT in patients with solid tumours:(1) to prevent CIT in patients without thrombocytopenia before chemotherapy, (2) to prevent recurrence of CIT, and (3) to treat CIT in patients with thrombocytopenia during chemotherapy.
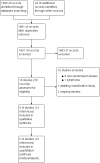
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL, to 28 September 2017), MEDLINE (from 1950 to 28 September 2017), as well as online registers of ongoing trials (Clinical Trials, Chinese Clinical Trial Register, Australian New Zealand Clinical Trial Registry, WHO ICTRP Search Portal, International Standard Randomised Controlled Trial Number registry, GlaxoSmithKline Clinical Study Register, and Amgen Clinical Trials) and conference proceedings (American Society of Hematology, American Society of Clinical Oncology, European Hematology Association, European Society of Medical Oncology, and Conference Proceedings Citation Index-Science, from 2002 up to September 2017) for studies.
Selection criteria: Randomised controlled trials (RCTs) comparing TPO-RAs alone, or in combination with other drugs, to placebo, no treatment, other drugs, or another TPO-RAs for CIT in patients with solid tumours.
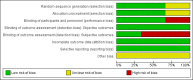
Data collection and analysis: Two review authors independently screened the results of the search strategies, extracted data, assessed risk of bias, and analysed data according to standard methodological methods expected by Cochrane.
Main results: We identified six trials eligible for inclusion, of which two are ongoing, and one awaiting classification study. The three included trials were conducted at many different sites in Europe, America, and Asia. All of the three studies recruited adult and elder participants (no children were included) with solid tumours, and compared TPO-RAs with placebo. No studies compared TPO-RAs alone, or in combination with other drugs, to no treatment, or other drugs, or another TPO-RAs.We judged the overall risk of bias as high as we found a high risk for detection bias. We assessed the risk of bias arising from inadequate blinding of outcome assessors as high for number and severity of bleeding episodes (one of the primary outcomes).To prevent CIT: We included two trials (206 participants) comparing TPO-RAs (eltrombopag, multiple-dose oral administration with chemotherapy) with placebo. The use of TPO-RAs may make little or no difference to the all-cause mortality at 33 weeks of follow-up (RR 1.35, 95% CI 0.53 to 3.45; one trial, 26 participants; low quality of evidence). There is not enough evidence to determine whether TPO-RAs reduce the number of patients with at least one bleeding episode of any severity (RR 0.62, 95% CI 0.22 to 1.78; two trials, 206 participants; very low quality of evidence). There is not enough evidence to determine whether TPO-RAs reduce the number of patients with at least one severe/life-threatening bleeding episode (RR 0.36, 95% CI 0.06 to 2.06; two trials, 206 participants; very low quality of evidence). No studies were found that looked at overall survival (one of the primary outcomes), the number of treatment cycles with at least one bleeding episode, the number of days on which bleeding occurred, the amount of bleeding, or quality of life.To prevent recurrence of CIT: We included one trial (62 participants) comparing TPO-RAs (romiplostim, single-dose subcutaneous administration with chemotherapy) with placebo. There is not enough evidence to determine whether TPO-RAs reduce the number of patients with at least one bleeding episode of any severity (RR 2.80, 95% CI 0.17 to 47.53; one trial, 62 participants; very low quality of evidence). There is not enough evidence to determine whether TPO-RAs reduce the number of patients with at least one severe/life-threatening bleeding episode (no severe/life-threatening bleeding episodes; one trial, 62 participants; very low quality of evidence). No studies were found that looked at overall survival (one of the primary outcomes), the number of treatment cycles with at least one bleeding episode, the number of days on which bleeding occurred, the amount of bleeding, or quality of life. We found one ongoing study (expected recruitment 74 participants), it is planned to give TPO-RAs (romiplostim, subcutaneous administration with chemotherapy) to participants, but to date this trial has not reported any outcomes.To treat CIT: We found one ongoing study (expected recruitment 83 participants), which is planned to give TPO-RAs (eltrombopag, seven days orally) to participants when their platelet counts are less than 75×109/L during chemotherapy. This trial was originally planned to complete in March 2017, however, the completion date has passed and no results are reported.The one awaiting classification study included patients without thrombocytopenia before chemotherapy (to prevent CIT), patients with thrombocytopenia during chemotherapy (to prevent recurrence of CIT), and other patients during chemotherapy (uncertain whether CIT had happened). There was no evidence for a difference in the number of patients with at least one bleeding episode of any severity (RR 0.27, 95% CI 0.07 to 1.02; one trial, 75 participants). There was no evidence for a difference in the number of patients with at least one severe/life-threatening bleeding episode (RR 0.44, 95% CI 0.03 to 6.77; one trial, 75 participants). This study did not address overall survival or quality of life.
Authors' conclusions: No certain conclusions can be drawn due to the lack of strong evidence in the review. The available weak evidence did not support the use of TPO-RAs for preventing CIT or preventing recurrence of CIT in patients with solid tumours. There was no evidence to support the use of TPO-RAs for treating CIT in patients with solid tumours.
Conflict of interest statement
Xia Zhang (XZ): none known
Yunhai Chuai (YC): none known
Wei Nie (WN): none known
Aiming Wang (AW): none known
Guanghai Dai (GD): none known
Figures
Update of
- doi: 10.1002/14651858.CD012035
Similar articles
-
Thrombopoietin mimetics for patients with myelodysplastic syndromes.Cochrane Database Syst Rev. 2017 Sep 30;9(9):CD009883. doi: 10.1002/14651858.CD009883.pub2. Cochrane Database Syst Rev. 2017. PMID: 28962071 Free PMC article.
-
Alternative agents to prophylactic platelet transfusion for preventing bleeding in people with thrombocytopenia due to chronic bone marrow failure: a meta-analysis and systematic review.Cochrane Database Syst Rev. 2016 Oct 31;10(10):CD012055. doi: 10.1002/14651858.CD012055.pub2. Cochrane Database Syst Rev. 2016. PMID: 27797129 Free PMC article.
-
Alternatives, and adjuncts, to prophylactic platelet transfusion for people with haematological malignancies undergoing intensive chemotherapy or stem cell transplantation.Cochrane Database Syst Rev. 2016 Aug 22;2016(8):CD010982. doi: 10.1002/14651858.CD010982.pub2. Cochrane Database Syst Rev. 2016. PMID: 27548292 Free PMC article.
-
Prophylactic platelet transfusions prior to surgery for people with a low platelet count.Cochrane Database Syst Rev. 2018 Sep 17;9(9):CD012779. doi: 10.1002/14651858.CD012779.pub2. Cochrane Database Syst Rev. 2018. PMID: 30221749 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
Cited by
-
Systematic literature review and meta-analysis on use of Thrombopoietic agents for chemotherapy-induced thrombocytopenia.PLoS One. 2022 Jun 9;17(6):e0257673. doi: 10.1371/journal.pone.0257673. eCollection 2022. PLoS One. 2022. PMID: 35679540 Free PMC article.
-
Thrombopoietin receptor agonists: ten years later.Haematologica. 2019 Jun;104(6):1112-1123. doi: 10.3324/haematol.2018.212845. Epub 2019 May 9. Haematologica. 2019. PMID: 31073079 Free PMC article. Review.
-
The Clinical Efficacy and Economic Benefits of Recombinant Human Thrombopoietin for the Treatment of Chemotherapy or Chemoradiotherapy-Induced Thrombocytopenia.Contrast Media Mol Imaging. 2022 Jul 14;2022:2256690. doi: 10.1155/2022/2256690. eCollection 2022. Contrast Media Mol Imaging. 2022. Retraction in: Contrast Media Mol Imaging. 2023 Jul 19;2023:9783232. doi: 10.1155/2023/9783232. PMID: 35909587 Free PMC article. Retracted.
-
Effects of thrombopoietin pre-treatment on peri-liver transplantation thrombocytopenia in a mouse model of cirrhosis with hypersplenism.World J Gastrointest Surg. 2023 Oct 27;15(10):2115-2122. doi: 10.4240/wjgs.v15.i10.2115. World J Gastrointest Surg. 2023. PMID: 37969704 Free PMC article.
-
Comparative Efficacy and Safety of Thrombopoietin Receptor Agonists in Adults With Thrombocytopenia: A Systematic Review and Network Meta-analysis of Randomized Controlled Trial.Front Pharmacol. 2021 Jul 28;12:704093. doi: 10.3389/fphar.2021.704093. eCollection 2021. Front Pharmacol. 2021. PMID: 34393785 Free PMC article.
References
References to studies included in this review
Kellum 2010 {published data only}
-
- 497115/003. A double‐blind, randomized, multicenter, placebo‐controlled, parallel group, dose ranging study to assess the efficacy, safety, and pharmacokinetics of an oral thrombopoietin receptor agonist (SB497115‐GR) administered at 50, 75, and 100 mg to cancer patients receiving multiple cycles of chemotherapy. GlaxoSmithKline (GSK) Clinical Study Register [accessed 28 January 2017].
-
- Bussel JB, McHutchison J, Provan D, Jagiello‐Gruzfeld A, Rafi R, Goodison S. Safety of eltrombopag, an oral non‐peptide platelet growth factor, in the treatment of thrombocytopenia: results of four randomized, placebo‐controlled studies. Blood 2007 Nov; Vol. 110, issue 11:1299.
-
- Hayes S, Mudd PN Jr, Ouellet D, Johnson BM, Williams D, Gibiansky E. Population PK/PD modeling of eltrombopag in subjects with advanced solid tumors with chemotherapy‐induced thrombocytopenia. Cancer Chemotherapy and Pharmacology 2013 Jun;71(6):1507‐20. - PubMed
-
- Kellum A, Jagiello‐Gruszfeld A, Bondarenko IN, Patwardhan R, Messam C, Mostafa Kamel Y. A randomized, double‐blind, placebo‐controlled, dose ranging study to assess the efficacy and safety of eltrombopag in patients receiving carboplatin/paclitaxel for advanced solid tumors. Current Medical Research and Opinion 2010 Oct;26(10):2339‐46. - PubMed
-
- Kellum A, Jagiello‐Gruszfeld A, Bondarenko IN, Rafi R, Giangiulio P, Messam C. Hepatobiliary (HB) safety of eltrombopag administered after carboplatin+paclitaxel (Carb+Pac) in patients (pts) with solid tumors. Journal of Clinical Oncology 2009; Vol. 27, issue suppl:abstr e20667.
Natale 2009 {published data only}
-
- 20050154. Dose/schedule finding trial of AMG 531 for chemotherapy‐induced thrombocytopenia (CIT) in non‐small cell lung cancer (NSCLC). Amgen Clinical Trials [accessed 28 January 2017].
-
- NCT00413283. Dose/schedule finding trial of romiplostim for chemotherapy‐induced thrombocytopenia (CIT) in non‐small cell lung cancer (NSCLC). ClinicalTrials.gov [accessed 28 January 2017].
-
- Natale R, Charu V, Schutte W, Albert I, Tehenes S, McCoy J, et al. Safety of romiplostim for treatment of chemotherapy‐induced thrombocytopenia (CIT) in patients with advanced non‐small cell lung cancer (NSCLC). European Journal of Cancer Supplements 2009 Sep;7(2):574.
Winer 2015 {published data only}
-
- 112765 (phase I). A randomized, blinded, placebo‐controlled, two‐phase, sequential cohort, dose finding study to assess the safety and efficacy of an oral thrombopoietin receptor agonist, eltrombopag (SB‐497115‐GR), administered to patients with solid tumors receiving gemcitabine monotherapy or the combination of gemcitabine plus carboplatin or cisplatin. GlaxoSmithKline (GSK) Clinical Study Register [accessed 28 January 2017].
-
- NCT01147809 (phase I). Safety and efficacy study for solid tumor patients treated with eltrombopag. ClinicalTrials.gov [accessed 28 January 2017].
-
- Winer ES, Safran H, Karaszewska B, Richards DA, Hartner L, Forget F, et al. Safety and efficacy of eltrombopag (epag) versus placebo (pbo) for the treatment (tx) of chemotherapy‐induced thrombocytopenia (CIT) in patients with solid tumors receiving gemcitabine (gem)‐based chemotherapy (ctx): A phase I study. Journal of Clinical Oncology 2012; Vol. 30, issue suppl:abstr 9117.
References to studies excluded from this review
Al‐Samkari 2016 {published data only}
-
- Al‐Samkari H, Kuter DJ, Goodarzi K, Marshall AL. Romiplostim for treatment and prevention of chemotherapy‐associated thrombocytopenia. Blood 2016;128(22):3748.
Chawla 2013 {published data only}
Dardis 2016 {published data only}
-
- Dardis C, Milton K, Ashby LS. Thrombopoietin receptor agonists are effective in treating chemotherapy‐induced thrombocytopenia, in patients with gliomas undergoing myelotoxic treatment. Neuro‐Oncology 2016;18(suppl 6):143‐4.
Iuliano 2016a {published data only}
-
- Iuliano F, Iuliano E, Perricelli A, Santoro MA, Pomillo A, Luci M, et al. Low doses of eltrombopag are safe and effective in the prophylaxis of the chemotherapy‐induced thrombocytopenia (CIT). Blood 2016;128(22):4926.
Iuliano 2016b {published data only}
-
- Iuliano F, Blotta S, Iuliano E, Santoro MA, Alessia P, Luci M, et al. Eltrombopag low doses as prophylaxis of the chemotherapy‐induced thrombocytopenia (CIT) in cancer patients treated with platinum based chemotherapy. Haematologica 2016;101(suppl 1):593.
Miao 2016 {published data only}
-
- Miao J, Leblebjian H, Fowler‐Scullion B, Parnes A. Single‐center experience with romiplostim for management of chemotherapy‐induced thrombocytopenia (CIT). Blood 2016;128(22):530. - PubMed
NCT02052882 {published data only}
-
- NCT02052882. An open label phase II study of romiplostim for chemotherapy induced thrombocytopenia. ClinicalTrials.gov [accessed 28 January 2017].
-
- Soff GA, Parameswaran R, Mantha S, Miao Y, Mones JV. Romiplostim management of chemotherapy‐induced thrombocytopenia. Journal of Clinical Oncology 2016;34(15 suppl):10134.
Urena 2016 {published data only}
-
- Urena LE, Jimenez DF, Morales ZM, Mohedo FH, Chacon, MJ. The role of romiplostim in chemotherapy‐induced thrombocytopenia treatment. Haematologica 2016;101(suppl 1):590.
Vadhan‐Raj 2009b {published data only}
-
- Vadhan‐Raj S, Trent J, Araujo DM, Patel S, Zhou X, Benjamin RS. Evaluation of AMG 531 in chemotherapy‐induced thrombocytopenia (CIT): Results of a phase I/II study. Journal of Clinical Oncology 2009;27(15 suppl):e20616.
Vadhan‐Raj 2010b {published data only}
-
- Vadhan‐Raj S, Hagemeister F, Fayad LE, Zhou X, ORoark SS, Ames K. Randomized, double‐blind, placebo‐controlled, dose and schedule‐finding study of AMG 531 In Chemotherapy‐Induced Thrombocytopenia (CIT): results of a phase I/II study. Blood 2010;116:1544.
References to studies awaiting assessment
Winer 2017 {published data only}
-
- 112765 (phase II). A randomized, blinded, placebo‐controlled, two‐phase, sequential cohort, dose finding study to assess the safety and efficacy of an oral thrombopoietin receptor agonist, eltrombopag (SB‐497115‐GR), administered to patients with solid tumors receiving gemcitabine monotherapy or the combination of gemcitabine plus carboplatin or cisplatin. GlaxoSmithKline (GSK) Clinical Study Register [accessed 28 January 2017].
-
- NCT01147809 (phase II). Safety and efficacy study for solid tumor patients treated with eltrombopag. ClinicalTrials.gov [accessed 28 January 2017].
-
- Winer ES, Safran H, Forget F, Karaszewska B, Bauer S, Khan D, et al. Safety and efficacy of eltrombopag (EPAG) vs placebo (PBO) for treatment of chemotherapy (CTx)‐induced thrombocytopenia (TCP) in patients (Pts) with solid tumors receiving gemcitabine (GEM)‐based CTx: A phase 2 study. Journal of Clinical Oncology 2015; Vol. 33, issue suppl:abstr 9602.
-
- Winer ES, Safran H, Karaszewska B, Bauer S, Khan D, Doerfel S, et al. Eltrombopag for thrombocytopenia in patients with advanced solid tumors receiving gemcitabine‐based chemotherapy: a randomized, placebo‐controlled phase 2 study. International Journal of Hematology 2017 Dec;106(6):765‐76. - PubMed
References to ongoing studies
EUCTR2013‐002564‐69‐DE {published data only}
-
- EUCTR2013‐002564‐69‐DE. Double‐blind, placebo‐controlled multicenter phase II trial to evaluate the efficacy and safety of romiplostim for the treatment of chemotherapy‐induced thrombocytopenia in subjects with relapsed ovarian cancer (2nd or further line). European Union Clinical Trials Register [accessed 28 September 2017].
NCT02093325 {published data only}
-
- NCT02093325. A randomized, double blind, placebo‐controlled study to assess the efficacy and safety of eltrombopag as a rescue of isolated chemotherapy‐induced thrombocytopenia in patients with gynecologic cancer. ClinicalTrials.gov [accessed 28 September 2017].
Additional references
AMGEN 2011
-
- Harper SE. Important information concerning updates to the US Nplate® (romiplostim). Prescribing Information 2011:http://www.amgen.com/pdfs/products/NPlate‐DHCP‐2011‐07‐27.pdf.pdf (accessed 10 April 2012).
Bai 2004
-
- Bai CM, Xu GX, Zhao YQ, Han SM, Shan YD. A multi‐center clinical trial of recombinant human thrombopoietin in the treatment of chemotherapy‐induced thrombocytopenia in patients with solid tumor. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2004;26(4):437‐41. - PubMed
Basser 2002
-
- Basser RL, O'Flaherty E, Green M, Edmonds M, Nichol J, Menchaca DM, et al. Development of pancytopenia with neutralizing antibodies to thrombopoietin after multicycle chemotherapy supported by megakaryocyte growth and development factor. Blood 2002;99(7):2599‐602. - PubMed
Bhandari 2004
Bhatia 2007
-
- Bhatia M, Davenport V, Cairo MS. The role of interleukin‐11 to prevent chemotherapy‐induced thrombocytopenia in patients with solid tumors, lymphoma, acute myeloid leukemia and bone marrow failure syndromes. Leukemia & Lymphoma 2007;48(1):9‐15. - PubMed
Bussel 2014
-
- Bussel JB, Lakkaraja M. Thrombopoietic agents: there is still much to learn. Presse Medicale 2014 Apr;43(4 Pt 2):e69‐78. - PubMed
Cairo 2005
-
- Cairo MS, Davenport V, Bessmertny O, Goldman SC, Berg SL, Kreissman SG, et al. Phase I/II dose escalation study of recombinant human interleukin‐11 following ifosfamide, carboplatin and etoposide in children, adolescents and young adults with solid tumours or lymphoma: a clinical, haematological and biological study. British Journal of Haematology 2005 Jan;128(1):49‐58. - PubMed
Cantor 2003
-
- Cantor SB, Elting LS, Hudson DV Jr, Rubenstein EB. Pharmacoeconomic analysis of oprelvekin (recombinant human interleukin‐11) for secondary prophylaxis of thrombocytopenia in solid tumour patients receiving chemotherapy. Cancer 2003 Jun;97(12):3099‐106. - PubMed
CSCO 2010
-
- Chinese Society of Clinical Oncology. Expert consensus on rational use of recombinant human IL‐11 for thrombocytopenia in solid tumor patients. Chinese Journal of Oncology 2010 Dec;32(12):948‐9. - PubMed
CSCO 2014
-
- Chinese Society of Clinical Oncology. Expert consensus on diagnosis and treatment of chemotherapy‐induced thrombocytopenia in cancer patients (2014 edition). Chinese Journal of Oncology 2014;36(11):876‐9. - PubMed
CTCAE 2009
-
- Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. 2009. The US National Cancer Institute (NCI).
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Desborough 2016
-
- Desborough M, Hadjinicolaou AV, Chaimani A, Trivella M, Vyas P, Doree C, et al. Alternative agents to prophylactic platelet transfusion for preventing bleeding in people with thrombocytopenia due to chronic bone marrow failure: a meta‐analysis and systematic review. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012055.pub2] - DOI - PMC - PubMed
Deutsch 2013
-
- Deutsch VR, Tomer A. Advances in megakaryocytopoiesis and thrombopoiesis: from bench to bedside. British Journal of Haematology 2013 Jun;161(6):778‐93. - PubMed
Dodillet 2017
Elting 2001
-
- Elting LS, Rubenstein EB, Martin CG, Kurtin D, Rodriguez S, Laiho E, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy‐induced thrombocytopenia. Journal of Clinical Oncology 2001;19(4):1137‐46. - PubMed
Elting 2003
-
- Elting LS, Cantor SB, Martin CG, Hamblin L, Kurtin D, Rivera E, et al. Cost of chemotherapy‐induced thrombocytopenia among patients with lymphoma or solid tumors. Cancer 2003;97(6):1541‐50. - PubMed
Erickson‐Miller 2005
-
- Erickson‐Miller CL, Delorme E, Tian SS, Hopson CB, Stark K, Giampa L, et al. Discovery and characterization of a selective, nonpeptidyl thrombopoietin receptor agonist. Experimental Hematology 2005;33(1):85‐93. - PubMed
Estcourt 2012
-
- Estcourt L, Stanworth S, Doree C, Hopewell S, Murphy MF, Tinmouth A, et al. Prophylactic platelet transfusion for prevention of bleeding in patients with haematological disorders after chemotherapy and stem cell transplantation. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD004269.pub3] - DOI - PMC - PubMed
Fletcher 2015
-
- Fletcher CH, DomBourian MG, Millward PA. Platelet transfusion for patients with cancer. Cancer Control 2015;22(1):47‐51. - PubMed
Hashiguchi 2015
-
- Hashiguchi Y, Fukuda T, Ichimura T, Matsumoto Y, Yasui T, Sumi T, et al. Chemotherapy‐induced thrombocytopenia and clinical bleeding in patients with gynecologic malignancy. European Journal of Gynaecological Oncology 2015;36(2):168‐73. - PubMed
Hassan 2011
-
- Hassan BA, Yusoff ZB, Hassali MA, Bin Othman S. Treatment patterns and outcomes in management of solid cancer patients suffering from thrombocytopenia in Penang hospital. Asian Pacific Journal of Cancer Prevention 2011;12(11):2841‐5. - PubMed
Higgins 2011a
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Higgins 2011c
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Hitchcock 2014
-
- Hitchcock IS, Kaushansky K. Thrombopoietin from beginning to end. British Journal of Haematology 2014 Apr;165(2):259‐68. - PubMed
Hoekman 1991
-
- Hoekman K, Wagstaff J, Groeningen CJ, Vermorken JB, Boven E, Pinedo HM. Effects of recombinant human granulocyte‐macrophage colony‐stimulating factor on myelosuppression induced by multiple cycles of high‐dose chemotherapy in patients with advanced breast cancer. Journal of the National Cancer Institute 1991;83(21):1546‐53. - PubMed
Kantarjian 2009
-
- Kantarjian H, Fenaux P, Sekeres MA, Becker PS, Boruchov A, Bowen D, et al. Safety and efficacy of romiplostim in patients with lower‐risk myelodysplastic syndrome and thrombocytopenia. Journal of Clinical Oncology 2009;28(3):437‐44. - PubMed
Kaufman 2015
-
- Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet transfusion: a clinical practice guideline from the AABB. Annals of Internal Medicine 2015 Feb;162(3):205‐13. - PubMed
Kuter 2008
-
- Kuter DJ. New drugs for familiar therapeutic targets: thrombopoietin receptor agonists and immune thrombocytopenic purpura. European Journal of Haematology 2008;80(Suppl 69):9‐18. - PubMed
Kuter 2013
-
- Kuter DJ. General aspects of thrombocytopenia, platelet transfusions, and thrombopoietic growth factors. In: Kitchens C, Kessler C, Konkle B editor(s). Consultative Hemostasis and Thrombosis. Philadelphia: Elsevier Saunders, 2013:103‐16.
Kuter 2015
-
- Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology (Williston Park) 2015 Apr;29(4):282‐94. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Lei 2006
-
- Lei W, Liang J, Chen WG, Ma XZ, Xu M, Du LL. Effectiveness and safety of recombinant human interleukin‐11 in the treatment of chemotherapy‐induced thrombocytopenia. Chinese Journal of Oncology 2006 Jul;28(7):542‐4. - PubMed
Levy 2008
-
- Levy B, Arnason JE, Bussel JB. The use of second‐generation thrombopoietic agents for chemotherapy‐induced thrombocytopenia. Current Opinion in Oncology 2008;20(6):690‐6. - PubMed
Li 2001
-
- Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood 2001;98(12):3241‐8. - PubMed
Li 2012
-
- Li Q, Ye M, Xiao W, Zhu J, Zhang S. Prophylactic recombinant human thrombopoietin treatment alleviates chemotherapy‐induced thrombocytopenia in tumor patients. Nan Fang Yi Ke Da Xue Xue Bao 2012;32(7):1064‐6. - PubMed
Machlus 2014
-
- Machlus KR, Thon JN, Italiano JE Jr. Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation. British Journal of Haematology 2014 Apr;165(2):227‐36. - PubMed
Parameswaran 2014
-
- Parameswaran R, Lunning M, Mantha S, Devlin S, Hamilton A, Schwartz G, et al. Romiplostim for management of chemotherapy‐induced thrombocytopenia. Supportive Care in Cancer 2014;22(5):1217‐22. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):15‐34. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rioux‐Massé 2013
-
- Rioux‐Massé B, Laroche V, Bowman RJ, Lindgren BR, Cohn CS, Pulkrabek SM, et al. The influence of bleeding on trigger changes for platelet transfusion in patients with chemotherapy‐induced thrombocytopenia. Transfusion 2013 Feb;53(2):306‐14. - PubMed
Schiffer 2001
-
- Schiffer CA, Anderson KC, Bennett CL, Bernstein S, Elting LS, Goldsmith M, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. Journal of Clinical Oncology 2001 Mar;19(5):1519‐38. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Sekeres 2011
-
- Sekeres MA, Kantarjian H, Fenaux P, Becker P, Boruchov A, Guerci‐Bresler A, et al. Subcutaneous or intravenous administration of romiplostim in thrombocytopenic patients with lower risk myelodysplastic syndromes. Cancer 2011;117(5):992‐1000. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Ten 2011
-
- Berg MJ, Bemt PM, Shantakumar S, Bennett D, Voest EE, Huisman A, et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: results from a retrospective hospital‐based cohort study. Drug Safety 2011 Dec;34(12):1151‐60. - PubMed
Tierney 2007
Vadhan‐Raj 2009a
-
- Vadhan‐Raj S. Management of chemotherapy‐induced thrombocytopenia: current status of thrombopoietic agents. Seminars in Hematology 2009;46(1 Suppl 2):S26‐32. - PubMed
Vadhan‐Raj 2010a
-
- Vadhan‐Raj S. Clinical findings with the first generation of thrombopoietic agents. Seminars in Hematology 2010 Jul;47(3):249‐57. - PubMed
Wandt 2014
Wang 2004
-
- Wang B, Nichol JL, Sullivan J T. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clinical Pharmacology and Therapeutics 2004;76(6):628‐38. - PubMed
Wang 2005
-
- Wang XY, Feng FY, Song ST, Wang HQ, Zhang MH, Liu J, et al. Phase II clinical trial on China manufactured recombinant human interleukin‐11 derivative in the prevention and treatment of chemotherapy‐induced thrombocytopenia. Chinese Journal of Oncology 2005 Jun;27(6):373‐6. - PubMed
Webert 2012
-
- Webert KE, Arnold DM, Lui Y, Carruthers J, Arnold E, Heddle NM. A new tool to assess bleeding severity in patients with chemotherapy‐induced thrombocytopenia (CME). Transfusion 2012 Nov;52(11):2466‐74. - PubMed
Wu 2009
-
- Wu Y, Aravind S, Ranganathan G, Martin A, Nalysnyk L. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: a descriptive study of a large outpatient oncology practice database, 2000‐2007. Clinical Therapeutic 2009;31 Pt 2:2416‐32. - PubMed
Wu 2010
-
- Wu Y, Cui X, Yuan Y, Zhao Z. Prevention and treatment of bone marrow suppression caused by antineoplastic agents. Drug Evaluation 2010;7(14):30‐6.
Wu 2012
-
- Wu S, Zhang Y, Xu L, Dai Y, Teng Y, Ma S, et al. Multicenter, randomized study of genetically modified recombinant human interleukin‐11 to prevent chemotherapy‐induced thrombocytopenia in cancer patients receiving chemotherapy. Supportive Care in Cancer 2012 Aug;20(8):1875‐84. - PubMed
Zeng 2011
Zhao 2001
-
- Zhao Y, Jiang J, Jiao L. Clinical tolerance test of recombinant human thrombopoietin. Zhong hua Yi Xue Za Zhi 2001;81(24):1508‐11. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources