TbSmee1 regulates hook complex morphology and the rate of flagellar pocket uptake in Trypanosoma brucei
- PMID: 29178204
- PMCID: PMC5777864
- DOI: 10.1111/mmi.13885
TbSmee1 regulates hook complex morphology and the rate of flagellar pocket uptake in Trypanosoma brucei
Abstract
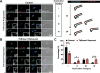
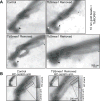
Trypanosoma brucei uses multiple mechanisms to evade detection by its insect and mammalian hosts. The flagellar pocket (FP) is the exclusive site of uptake from the environment in trypanosomes and shields receptors from exposure to the host. The FP neck is tightly associated with the flagellum via a series of cytoskeletal structures that include the hook complex (HC) and the centrin arm. These structures are implicated in facilitating macromolecule entry into the FP and nucleating the flagellum attachment zone (FAZ), which adheres the flagellum to the cell surface. TbSmee1 (Tb927.10.8820) is a component of the HC and a putative substrate of polo-like kinase (TbPLK), which is essential for centrin arm and FAZ duplication. We show that depletion of TbSmee1 in the insect-resident (procyclic) form of the parasite causes a 40% growth decrease and the appearance of multinucleated cells that result from defective cytokinesis. Cells lacking TbSmee1 contain HCs with aberrant morphology and show delayed uptake of both fluid-phase and membrane markers. TbPLK localization to the tip of the new FAZ is also blocked. These results argue that TbSmee1 is necessary for maintaining HC morphology, which is important for the parasite's ability to take up molecules from its environment.
© 2017 John Wiley & Sons Ltd.
Figures








Similar articles
-
BOH1 cooperates with Polo-like kinase to regulate flagellum inheritance and cytokinesis initiation in Trypanosoma brucei.J Cell Sci. 2019 Jul 15;132(14):jcs230581. doi: 10.1242/jcs.230581. J Cell Sci. 2019. PMID: 31217284 Free PMC article.
-
Characterisation of TbSmee1 suggests endocytosis allows surface-bound cargo to enter the trypanosome flagellar pocket.J Cell Sci. 2023 Oct 15;136(20):jcs261548. doi: 10.1242/jcs.261548. Epub 2023 Oct 26. J Cell Sci. 2023. PMID: 37737012 Free PMC article.
-
A Novel Basal Body Protein That Is a Polo-like Kinase Substrate Is Required for Basal Body Segregation and Flagellum Adhesion in Trypanosoma brucei.J Biol Chem. 2015 Oct 9;290(41):25012-22. doi: 10.1074/jbc.M115.674796. Epub 2015 Aug 13. J Biol Chem. 2015. PMID: 26272611 Free PMC article.
-
Cell-to-flagellum attachment and surface architecture in kinetoplastids.Trends Parasitol. 2023 May;39(5):332-344. doi: 10.1016/j.pt.2023.02.009. Epub 2023 Mar 16. Trends Parasitol. 2023. PMID: 36933967 Review.
-
The Flagellum Attachment Zone: 'The Cellular Ruler' of Trypanosome Morphology.Trends Parasitol. 2016 Apr;32(4):309-324. doi: 10.1016/j.pt.2015.12.010. Epub 2016 Jan 8. Trends Parasitol. 2016. PMID: 26776656 Free PMC article. Review.
Cited by
-
Bhalin, an Essential Cytoskeleton-Associated Protein of Trypanosoma brucei Linking TbBILBO1 of the Flagellar Pocket Collar with the Hook Complex.Microorganisms. 2021 Nov 11;9(11):2334. doi: 10.3390/microorganisms9112334. Microorganisms. 2021. PMID: 34835460 Free PMC article.
-
miR-204-5p Represses Bone Metastasis via Inactivating NF-κB Signaling in Prostate Cancer.Mol Ther Nucleic Acids. 2019 Dec 6;18:567-579. doi: 10.1016/j.omtn.2019.09.008. Epub 2019 Sep 18. Mol Ther Nucleic Acids. 2019. PMID: 31678733 Free PMC article.
-
The trypanosome-specific proteins FPRC and CIF4 regulate cytokinesis initiation by recruiting CIF1 to the cytokinesis initiation site.J Biol Chem. 2019 Nov 8;294(45):16672-16683. doi: 10.1074/jbc.RA119.010538. Epub 2019 Sep 20. J Biol Chem. 2019. PMID: 31540971 Free PMC article.
-
BOH1 cooperates with Polo-like kinase to regulate flagellum inheritance and cytokinesis initiation in Trypanosoma brucei.J Cell Sci. 2019 Jul 15;132(14):jcs230581. doi: 10.1242/jcs.230581. J Cell Sci. 2019. PMID: 31217284 Free PMC article.
-
Characterisation of TbSmee1 suggests endocytosis allows surface-bound cargo to enter the trypanosome flagellar pocket.J Cell Sci. 2023 Oct 15;136(20):jcs261548. doi: 10.1242/jcs.261548. Epub 2023 Oct 26. J Cell Sci. 2023. PMID: 37737012 Free PMC article.
References
-
- Absalon S, Blisnick T, Bonhivers M, Kohl L, Cayet N, Toutirais G, et al. Flagellum elongation is required for correct structure, orientation and function of the flagellar pocket in Trypanosoma brucei. Journal of Cell Science. 2008;121:3704–3716. - PubMed
-
- Alexander DL, Schwartz KJ, Balber AE, Bangs JD. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J Cell Sci. 2002;115:3253–3263. - PubMed
-
- André J, Harrison S, Towers K, Qi X, Vaughan S, McKean PG, Ginger ML. The tubulin cofactor C family member TBCCD1 orchestrates cytoskeletal filament formation. Journal of Cell Science. 2013;126:5350–5356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

