Antibiotic-loaded Bone Cement as Prophylaxis in Total Joint Replacement
- PMID: 29178309
- PMCID: PMC6584364
- DOI: 10.1111/os.12351
Antibiotic-loaded Bone Cement as Prophylaxis in Total Joint Replacement
Abstract
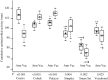
One of its most serious complications associated with arthroplasty is the development of infections. Although its prevalence is only between 0.5% and 3%, in some cases it can lead to death. Therefore, an important challenge in joint surgery is the prevention of infections when an arthroplasty is performed. The use of antibiotic-loaded cements could be a suitable tool due to numerous advantages. The main advantage of the use of antibiotic loading into bone cement derives directly from antibiotic release in the effect site, allowing achievement of high concentrations at the site of action, and minimal or no systemic toxicity. This route of administration was first described by Buchholz and Engelbrecht. In the case of infection treatment, this is an established method and its good results have been confirmed. However, its role in infection prevention, and, therefore, the use of these systems in clinical practice, has proved controversial because of the uncertainty about the development of possible antibiotic resistance after prolonged exposure time, their effectiveness, the cost of the systems, toxicity and loosening of mechanical properties. This review discusses all these topics, focusing on effectiveness and safety, antibiotic decisions, cement type, mixing method, release kinetics and future perspectives. The final objective is to provide the orthopaedic surgeons the right information in their clinical practice based on current evidence.
Keywords: Antibiotic; Arthroplasty; Bioactivity; Bone cement; Elution kinetics.
© 2017 Chinese Orthopaedic Association and John Wiley & Sons Australia, Ltd.
Figures
References
-
- Regis D, Sandri A, Samaila E, Benini A, Bondi M, Magnan B. Release of gentamicin and vancomycin from preformed spacers in infected total hip arthroplasties: measurement of concentrations and inhibitory activity in patients’ drainage fluids and serum. ScientificWorldJournal, 2013, 2013: 752184. - PMC - PubMed
-
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two‐stage revision of infected hip arthroplasty. J Trauma, 2009, 66: 804–808. - PubMed
-
- Buchholz HW, Engelbrecht H. Depot effects of various antibiotics mixed with Palacos resins. Chirurg, 1970, 41: 511–515. - PubMed
-
- Arciola CR, Campoccia D, Montanaro L. Effects on antibiotic resistance of Staphylococcus epidermidis following adhesion to polymethylmethacrylate and to silicone surfaces. Biomaterials, 2002, 23: 1495–1502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical