FOXO protects against age-progressive axonal degeneration
- PMID: 29178390
- PMCID: PMC5771393
- DOI: 10.1111/acel.12701
FOXO protects against age-progressive axonal degeneration
Abstract
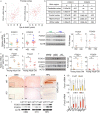
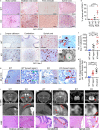
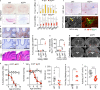
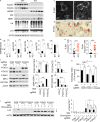
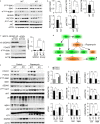
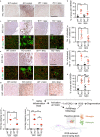
Neurodegeneration resulting in cognitive and motor impairment is an inevitable consequence of aging. Little is known about the genetic regulation of this process despite its overriding importance in normal aging. Here, we identify the Forkhead Box O (FOXO) transcription factor 1, 3, and 4 isoforms as a guardian of neuronal integrity by inhibiting age-progressive axonal degeneration in mammals. FOXO expression progressively increased in aging human and mouse brains. The nervous system-specific deletion of Foxo transcription factors in mice accelerates aging-related axonal tract degeneration, which is followed by motor dysfunction. This accelerated neurodegeneration is accompanied by levels of white matter astrogliosis and microgliosis in middle-aged Foxo knockout mice that are typically only observed in very old wild-type mice and other aged mammals, including humans. Mechanistically, axonal degeneration in nerve-specific Foxo knockout mice is associated with elevated mTORC1 activity and accompanying proteotoxic stress due to decreased Sestrin3 expression. Inhibition of mTORC1 by rapamycin treatment mimics FOXO action and prevented axonal degeneration in Foxo knockout mice with accelerated nervous system aging. Defining this central role for FOXO in neuroprotection during mammalian aging offers an invaluable window into the aging process itself.
Keywords: FOXO; accelerated aging; aging; central nervous system; mouse models; neurodegeneration; neuroinflammation; oxidative stress.
© 2017 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures
References
-
- Bodles, A. M. , & Barger, S. W. (2004). Cytokines and the aging brain ‐ what we don't know might help us. Trends in Neurosciences, 27, 621–626. https://doi.org/10.1016/j.tins.2004.07.011 - DOI - PubMed
-
- Bronson, R. T. , Lipman, R. D. , & Harrison, D. E. (1993). Age‐related gliosis in the white matter of mice. Brain Research, 609, 124–128. https://doi.org/10.1016/0006-8993(93)90864-J - DOI - PubMed
-
- Caccamo, A. , Majumder, S. , Richardson, A. , Strong, R. , & Oddo, S. (2010). Molecular interplay between mammalian target of rapamycin (mTOR), amyloid‐beta, and Tau: Effects on cognitive impairments. Journal of Biological Chemistry, 285, 13107–13120. https://doi.org/10.1074/jbc.M110.100420 - DOI - PMC - PubMed
-
- Calixto, A. , Jara, J. S. , & Court, F. A. (2012). Diapause formation and downregulation of insulin‐like signaling via DAF‐16/FOXO delays axonal degeneration and neuronal loss. PLoS Genetics, 8, e1003141 https://doi.org/10.1371/journal.pgen.1003141 - DOI - PMC - PubMed
-
- Chen, C. C. , Jeon, S. M. , Bhaskar, P. T. , Nogueira, V. , Sundararajan, D. , Tonic, I. , … Hay, N. (2010). FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Developmental Cell, 18, 592–604. https://doi.org/10.1016/j.devcel.2010.03.008 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

