Pleural plaques in lung cancer screening by low-dose computed tomography: prevalence, association with lung cancer and mortality
- PMID: 29178853
- PMCID: PMC5702182
- DOI: 10.1186/s12890-017-0506-3
Pleural plaques in lung cancer screening by low-dose computed tomography: prevalence, association with lung cancer and mortality
Abstract
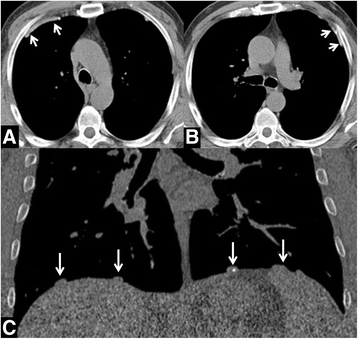
Background: To report the prevalence of pleural plaques in a lung cancer screening trial by low-dose computed tomography (LDCT) and to test the association with incidence of lung cancer and mortality.
Methods: The LDCT of 2303 screenees were retrospectively reviewed with the specific aim of describing the prevalence and features of pleural plaques. Self-administered questionnaire was used to assess asbestos exposure. Frequency of lung cancer, lung cancer mortality, and overall mortality were detailed according to presence of pleural findings. Statistical analyses included comparison of mean or median, contingency tables, and Cox model for calculation of hazard ratio (HR) and its 95% confidence interval (CI).
Results: Among male screenees, 31/1570 (2%) showed pleural abnormalities, 128/1570 (8.2%) disclosed asbestos exposure, 23/31 (74.2%) subjects with pleural plaques consistently denied exposure to asbestos. There was a trend for higher frequency of lung cancer among subjects with pleural plaques (9.7% vs 4.2%). Lung cancer in subjects with pleural plaques was always diagnosed in advanced stage. Subjects with pleural plaques showed HR 5.48 (95% CI 1.61-18.70) for mortality from lung cancer.
Conclusions: Pleural plaques are a risk factor for lung cancer mortality that can be detected in lung cancer screening by LDCT, also in subjects that are not aware of asbestos exposure.
Trial registration: NCT02837809 - Retrospectively registered July 1, 2016 - Enrolment of first participant September 2005.
Keywords: Asbestos exposure; Lung cancer screening; Pleural abnormalities; Pleural plaques; Post-test refinement of individual risk; Self-disclosure of asbestos exposure.
Conflict of interest statement
Ethics approval and consent to participate
The Institutional Review Board of the Fondazione IRCCS Istituto Nazionale dei Tumori di Milano approved the Multicenter Italian Lung Detection (MILD) protocol, and written informed consent was obtained from all participants. Informed consent included retrospective evaluation of MILD data, as performed in this study. Therefore, specific consent was waived for this study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests..
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

