SH003 suppresses breast cancer growth by accumulating p62 in autolysosomes
- PMID: 29179443
- PMCID: PMC5687613
- DOI: 10.18632/oncotarget.11393
SH003 suppresses breast cancer growth by accumulating p62 in autolysosomes
Abstract
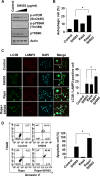
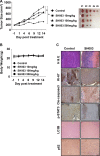
Drug markets revisits herbal medicines, as historical usages address their therapeutic efficacies with less adverse effects. Moreover, herbal medicines save both cost and time in development. SH003, a modified version of traditional herbal medicine extracted from Astragalus membranaceus (Am), Angelica gigas (Ag), and Trichosanthes Kirilowii Maximowicz (Tk) with 1:1:1 ratio (w/w) has been revealed to inhibit tumor growth and metastasis on highly metastatic breast cancer cells, both in vivo and in vitro with no toxicity. Meanwhile, autophagy is imperative for maintenance cellular homeostasis, thereby playing critical roles in cancer progression. Inhibition of autophagy by pharmacological agents induces apoptotic cell death in cancer cells, resulting in cancer treatment. In this study, we demonstrate that SH003-induced autophagy via inhibiting STAT3 and mTOR results in an induction of lysosomal p62/SQSTM1 accumulation-mediated reactive oxygen species (ROS) generation and attenuates tumor growth. SH003 induced autophagosome and autolysosome formation by inhibiting activation of STAT3- and mTOR-mediated signaling pathways. However, SH003 blocked autophagy-mediated p62/SQSTM1 degradation through reducing of lysosomal proteases, Cathepsins, resulting in accumulation of p62/SQSTM1 in the lysosome. The accumulation of p62/SQSTM1 caused the increase of ROS, which resulted in the induction of apoptotic cell death. Therefore, we conclude that SH003 suppresses breast cancer growth by inducing autophagy. In addition, SH003-induced p62/SQSTM1 could function as an important mediator for ROS generation-dependent cell death suggesting that SH003 may be useful for treating breast cancer.
Keywords: SH003; apoptosis; autophagy; breast cancer; p62.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that they no conflicts interests.
Figures







References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

