Targeting epigenetics for treatment of BRAF mutated metastatic melanoma with decitabine in combination with vemurafenib: A phase lb study
- PMID: 29179510
- PMCID: PMC5687680
- DOI: 10.18632/oncotarget.21269
Targeting epigenetics for treatment of BRAF mutated metastatic melanoma with decitabine in combination with vemurafenib: A phase lb study
Abstract
Introduction: Epigenetic modifications play an important role in progression and development of resistance in V600EBRAF positive metastatic melanoma. Therefore, we hypothesized that the action of vemurafenib (BRAF inhibitor) can be made more effective by combining with low dose decitabine (a DNA methyltransferase inhibitor). The primary objective of this phase lb study was to determine the dose limiting toxicity and maximum tolerated dose of combination of subcutaneous decitabine with oral vemurafenib in patients with V600EBRAF positive metastatic melanoma with or without any prior treatment.
Experimental design: The study employed 3+3 dose escalation combining subcutaneous decitabine at different doses and schedules (4 cohorts) with the standard oral dose of vemurafenib 960 mg twice daily. Preclinical assessment and further analysis were also performed in A375 melanoma cell line.
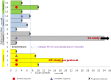
Results: Fourteen patients received study treatment. No dose limiting toxicity was encountered and maximum tolerated dose was not reached. Important toxicities included fatigue, increased creatinine, neutropenia, leucopenia, hypophosphatemia, rash and hyperuricemia. Three patients achieved complete response, three had partial response and five had stable disease. Preclinical assessment demonstrated action of the combination which delayed the development of acquired resistance and improved duration of treatment sensitivity.
Conclusions: The combination of oral vemurafenib with subcutaneous decitabine is safe and showed activity in V600EBRAF positive metastatic melanoma. Since most responses were seen in cohort 1, which utilized low-dose, long-term decitabine, future studies of this combination treatment should utilize longer duration of decitabine, at the lowest dose of 0.1 mg/kg.
Keywords: BRAF; decitabine; epigenetics; melanoma; vemurafenib.
Conflict of interest statement
CONFLICTS OF INTEREST YZ has no direct competing interest to the study. He has institutional research support from NewLink. He is on the advisory board of Roche Diagnostics, EISAI and Castle. VM also has no direct competing interest to the study. He has research support from Immunocellular, Orbus Therapeutics and New Link Genetics. MH is founder and stockholder of SynderBio, Inc. MM is on the advisory board of Genentech, BMS, EISAI, Novartis and EMD Serono. Other authors didn’t report any competing interest.
Figures





References
-
- Ugurel S, Rohmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A, Larkin J, Long GV, Lorigan P, McArthur GA, Ribas A, Robert C, Schadendorf D, et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies. Eur J Cancer. 2016;53:125–34. https://doi.org/10.1016/j.ejca.2015.09.013. - DOI - PubMed
-
- Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M, Palmieri G, Testori A, Marincola FM, Mozzillo N. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. https://doi.org/10.1186/1479-5876-10-85. - DOI - PMC - PubMed
-
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, et al. BRIM-3 Study Group Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. https://doi.org/10.1056/NEJMoa1103782. - DOI - PMC - PubMed
-
- McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, Ribas A, Hogg D, Hamid O, Ascierto PA, Garbe C, Testori A, Maio M, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–32. https://doi.org/10.1016/S1470-2045(14)70012-9. - DOI - PMC - PubMed
-
- Spagnolo F, Ghiorzo P, Queirolo P. Overcoming resistance to BRAF inhibition in BRAF-mutated metastatic melanoma. Oncotarget. 2014;5:10206–21. https://doi.org/10.18632/oncotarget.2602. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

