Erythropoietin Attenuates the Brain Edema Response after Experimental Traumatic Brain Injury
- PMID: 29179621
- PMCID: PMC5806078
- DOI: 10.1089/neu.2017.5015
Erythropoietin Attenuates the Brain Edema Response after Experimental Traumatic Brain Injury
Abstract
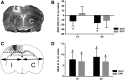
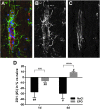
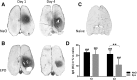
Erythropoietin (EPO) has neuroprotective effects in multiple central nervous system (CNS) injury models; however EPO's effects on traumatic brain edema are elusive. To explore EPO as an intervention in traumatic brain edema, male Sprague-Dawley (SD) rats were subjected to blunt, controlled traumatic brain injury (TBI). Animals were randomized to EPO 5000 IU/kg or saline (control group) intraperitoneally within 30 min after trauma and once daily for 4 consecutive days. Brain MRI, immunohistofluorescence, immunohistochemistry, and quantitative protein analysis were performed at days 1 and 4 post- trauma. EPO significantly prevented the loss of the tight junction protein zona occludens 1 (ZO-1) observed in control animals after trauma. The decrease of ZO-1 in the control group was associated with an immunoglobulin (Ig)G increase in the perilesional parenchyma, indicating blood-brain barrier (BBB) dysfunction and increased permeability. EPO treatment attenuated decrease in apparent diffusion coefficient (ADC) after trauma, suggesting a reduction of cytotoxic edema, and reduced the IgG leakage, indicating that EPO contributed to preserve BBB integrity and attenuated vasogenic edema. Animals treated with EPO demonstrated conserved levels of aquaporin 4 (AQP4) protein expression in the perilesional area, whereas control animals showed a reduction of AQP4. We show that post TBI administration of EPO decreases early cytotoxic brain edema and preserves structural and functional properties of the BBB, leading to attenuation of the vasogenic edema response. The data support that the mechanisms involve preservation of the tight junction protein ZO-1 and the water channel AQP4, and indicate that treatment with EPO may have beneficial effects on the brain edema response following TBI.
Keywords: BBB; EPO; TBI; aquaporin; brain edema.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Coronado V.G., McGuire L.C., Sarmiento K., Bell J., Lionbarger M.R., Jones C.D., Geller A.I., Khoury N., and Xu L. ( 2012). Trends in Traumatic Brain Injury in the U.S. and the public health response: 1995–2009. J. Safety Res. 43, 299– 307 - PubMed
-
- Coronado V.G., Haileyesus T., Cheng T.A., Bell J.M., Haarbauer-Krupa J., Lionbarger M.R., Flores-Herrera J., McGuire L.C., and Gilchrist J. ( 2015). Trends in sports- and recreation-related traumatic brain injuries treated in US emergency departments: The National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP) 2001–2012. J. Head Trauma Rehabil. 30, 185– 197 - PMC - PubMed
-
- Clausen T., and Bullock R. ( 2001). Medical treatment and neuroprotection in traumatic brain injury. Curr. Pharm. Des. 7, 1517– 1532 - PubMed
-
- Faul M., Wald M.M., Rutland-Brown W., Sullivent E.E., and Sattin R.W. ( 2007). Using a cost-benefit analysis to estimate outcomes of a clinical treatment guideline: testing theBrain Trauma Foundation guidelines for the treatment of severe traumatic brain injury. J. Trauma 63, 1271– 1278 - PubMed
-
- Honeybul S. ( 2011). An update on the management of traumatic brain injury. J. Neurosurg. Sci. 55, 343– 355 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
