Aminoglucose-functionalized, redox-responsive polymer nanomicelles for overcoming chemoresistance in lung cancer cells
- PMID: 29179722
- PMCID: PMC5704373
- DOI: 10.1186/s12951-017-0316-z
Aminoglucose-functionalized, redox-responsive polymer nanomicelles for overcoming chemoresistance in lung cancer cells
Abstract
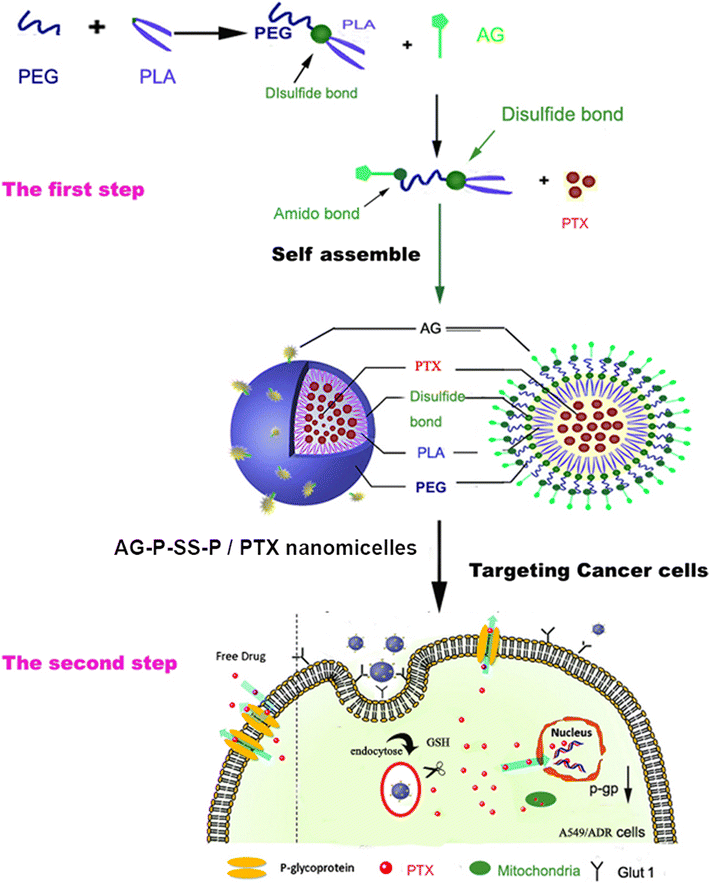
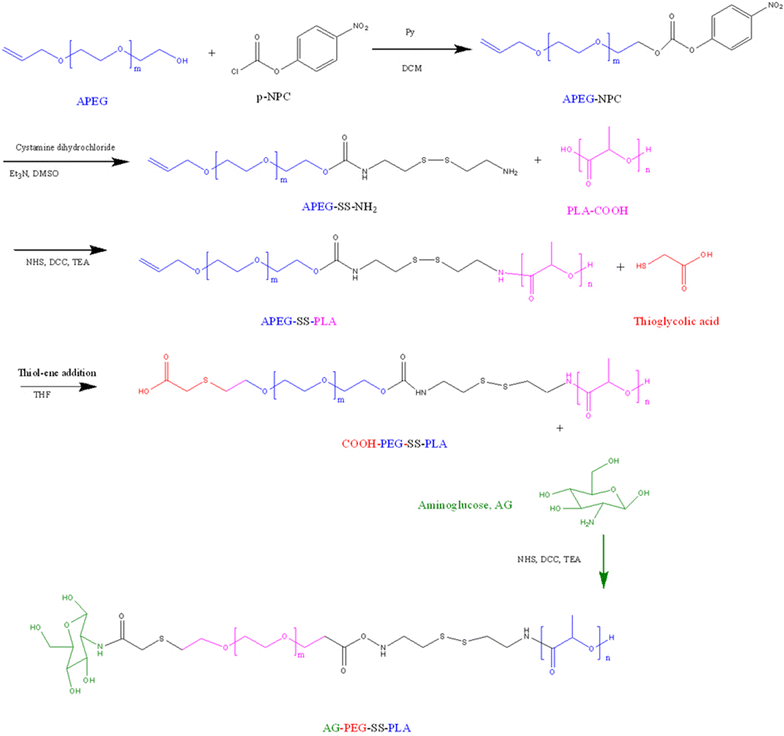
Background: Chemotherapeutic drugs used for cancer therapy frequently encounter multiple-drug resistance (MDR). Nanoscale carriers that can target tumors to accumulate and release drugs intracellularly have the greatest potential for overcoming MDR. Glucose transporter-1 (GLUT-1) and glutathione (GSH) overexpression in cancer cells was exploited to assemble aminoglucose (AG)-conjugated, redox-responsive nanomicelles from a single disulfide bond-bridged block polymer of polyethylene glycol and polylactic acid (AG-PEG-SS-PLA). However, whether this dual functional vector can overcome MDR in lung cancer is unknown.
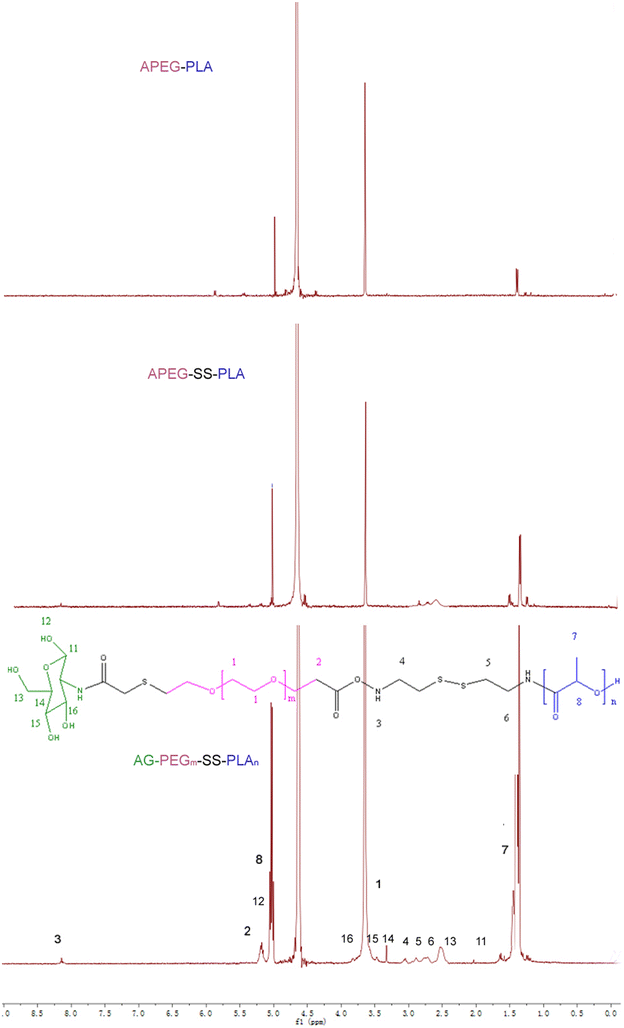
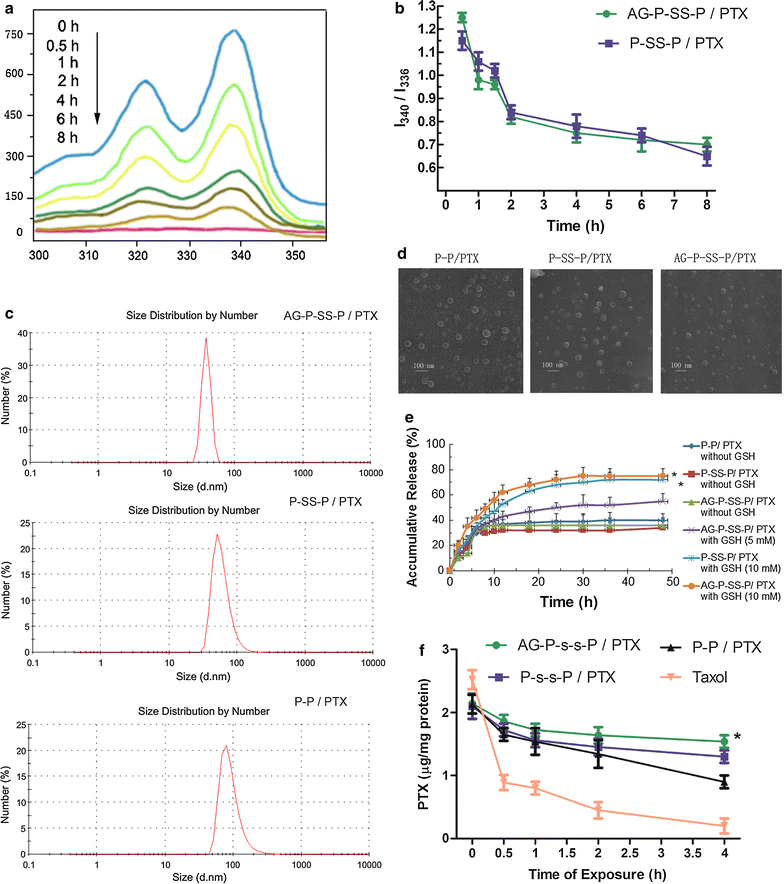
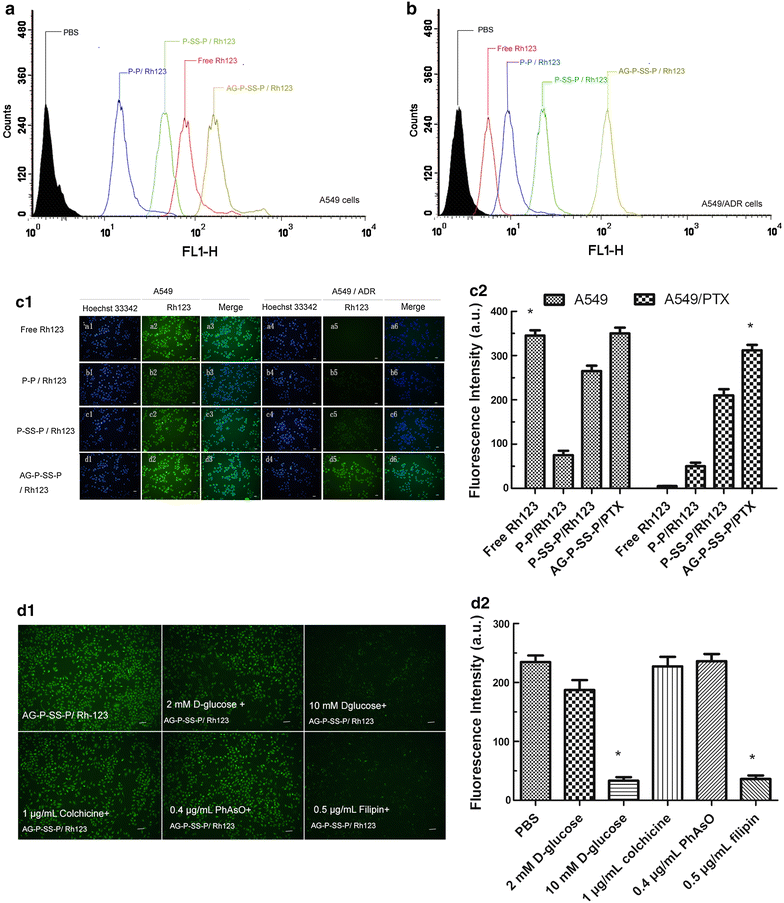
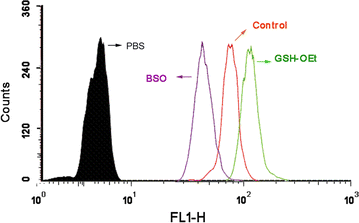
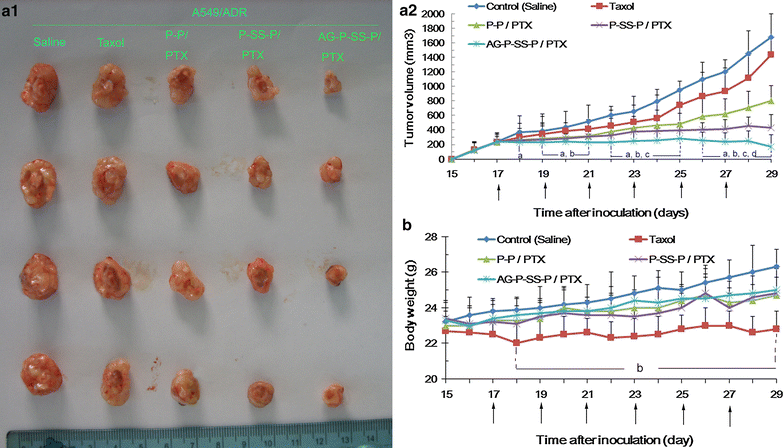
Results: In this experiment, AG-PEG-SS-PLA was synthetized successfully, and paclitaxel (PTX)-loaded AG-PEG-SS-PLA (AG-PEG-SS-PLA/PTX) nanomicelles exhibited excellent physical properties. These nanomicelles show enhanced tumor targeting as well as drug accumulation and retention in MDR cancer cells. Caveolin-dependent endocytosis is mainly responsible for nanomicelle internalization. After internalization, the disulfide bond of AG-PEG-SS-PLA is cleaved in the presence of high intracellular glutathione levels, causing the hydrophobic core to become a polar aqueous solution, which subsequently results in nanomicelle disassembly and the rapid release of encapsulated PTX. Reduced drug resistance was observed in cancer cells in vitro. The caspase-9 and caspase-3 cascade was activated by the AG-PEG-SS-PLA/PTX nanomicelles through upregulation of the pro-apoptotic proteins Bax and Bid and suppression of the anti-apoptotic protein Bcl-2, thereby increasing apoptosis. Furthermore, significantly enhanced tumor growth inhibition was observed in nude mice bearing A549/ADR xenograft tumors after the administration of AG-PEG-SS-PLA/PTX nanomicelles via tail injection.
Conclusions: These promising results indicate that AG-PEG-SS-PLA/PTX nanomicelles could provide the foundation for a paradigm shift in MDR cancer therapy.
Keywords: Cancer therapy; Gult-1; Multidrug resistance; Nanomicelles; Redox-responsive polymer.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

