Laser-modified titanium surfaces enhance the osteogenic differentiation of human mesenchymal stem cells
- PMID: 29179738
- PMCID: PMC5704576
- DOI: 10.1186/s13287-017-0717-9
Laser-modified titanium surfaces enhance the osteogenic differentiation of human mesenchymal stem cells
Abstract
Background: Titanium surfaces have been modified by various approaches with the aim of improving the stimulation of osseointegration. Laser beam (Yb-YAG) treatment is a controllable and flexible approach to modifying surfaces. It creates a complex surface topography with micro and nano-scaled patterns, and an oxide layer that can improve the osseointegration of implants, increasing their usefulness as bone implant materials.
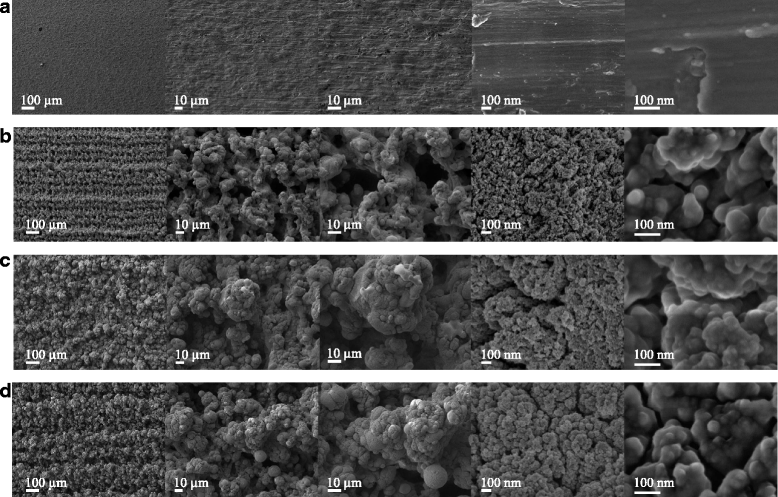
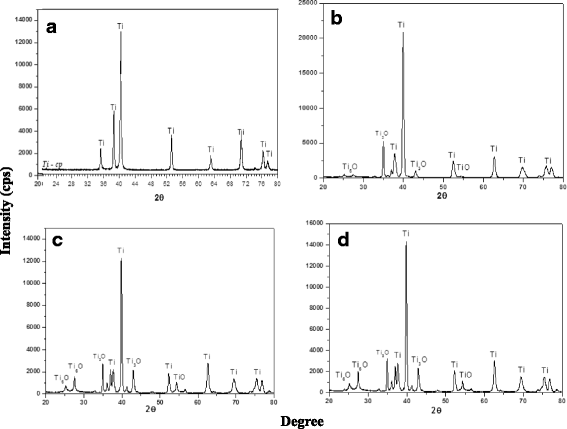
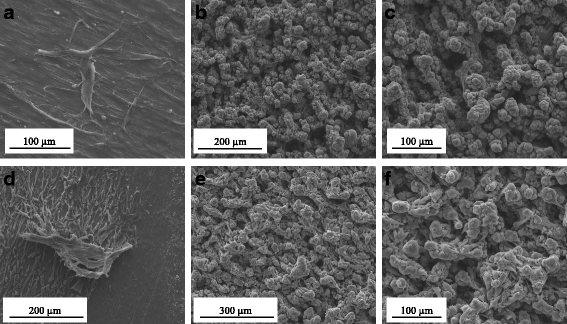
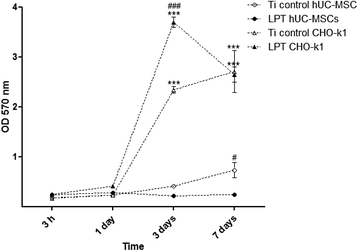
Methods: Laser beam irradiation at various fluences (132, 210, or 235 J/cm2) was used to treat commercially pure titanium discs to create complex surface topographies. The titanium discs were investigated by scanning electron microscopy, X-ray diffraction, and measurement of contact angles. The surface generated at a fluence of 235 J/cm2 was used in the biological assays. The behavior of mesenchymal stem cells from an umbilical cord vein was evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, a mineralization assay, and an alkaline phosphatase activity assay and by carrying out a quantitative real-time polymerase chain reaction for osteogenic markers. CHO-k1 cells were also exposed to titanium discs in the MTT assay.
Results: The best titanium surface was that produced by laser beam irradiation at 235 J/cm2 fluence. Cell proliferation analysis revealed that the CHO-k1 and mesenchymal stem cells behaved differently. The laser-processed titanium surface increased the proliferation of CHO-k1 cells, reduced the proliferation of mesenchymal stem cells, upregulated the expression of the osteogenic markers, and enhanced alkaline phosphatase activity.
Conclusions: The laser-treated titanium surface modulated cellular behavior depending on the cell type, and stimulated osteogenic differentiation. This evidence supports the potential use of laser-processed titanium surfaces as bone implant materials, and their use in regenerative medicine could promote better outcomes.
Keywords: Biocompatibility; Human umbilical cord; Laser beam (Yb-YAG); Mesenchymal stem cells; Osteoinduction; Surface modification; Titanium.
Conflict of interest statement
Ethics approval and consent to participate
This work was submitted to and approved by the Ethics Committee of the Federal University of Rio Grande do Norte (FR132464). Umbilical cord specimens were obtained after written informed consent was signed by mothers.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Geetha M, Singh AK, Asokamani R, Gogia AK. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Prog Mater Sci. 2009;54:397–425. doi: 10.1016/j.pmatsci.2008.06.004. - DOI
-
- Novin M, Faghihi S. Mouse bone marrow-derived mesenchymal stem cell response to nanostructured titanium substrates produced by high-pressure torsion. Surf Interface Anal. 2013;45:619–27. doi: 10.1002/sia.5101. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

