Neuroimaging genomics in psychiatry-a translational approach
- PMID: 29179742
- PMCID: PMC5704437
- DOI: 10.1186/s13073-017-0496-z
Neuroimaging genomics in psychiatry-a translational approach
Abstract
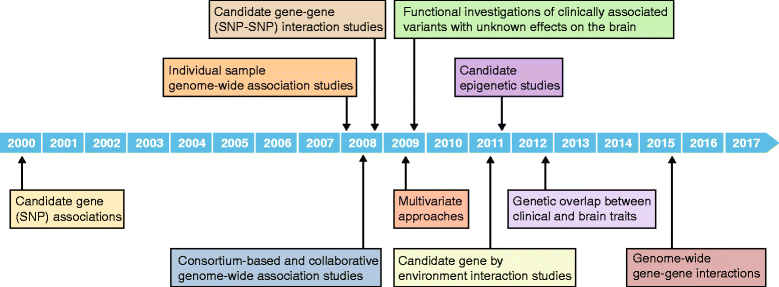
Neuroimaging genomics is a relatively new field focused on integrating genomic and imaging data in order to investigate the mechanisms underlying brain phenotypes and neuropsychiatric disorders. While early work in neuroimaging genomics focused on mapping the associations of candidate gene variants with neuroimaging measures in small cohorts, the lack of reproducible results inspired better-powered and unbiased large-scale approaches. Notably, genome-wide association studies (GWAS) of brain imaging in thousands of individuals around the world have led to a range of promising findings. Extensions of such approaches are now addressing epigenetics, gene-gene epistasis, and gene-environment interactions, not only in brain structure, but also in brain function. Complementary developments in systems biology might facilitate the translation of findings from basic neuroscience and neuroimaging genomics to clinical practice. Here, we review recent approaches in neuroimaging genomics-we highlight the latest discoveries, discuss advantages and limitations of current approaches, and consider directions by which the field can move forward to shed light on brain disorders.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kovelman I. Neuroimaging methods. In: Hoff E, editor. Research methods in child language: a practical guide. Oxford, UK: Wiley-Blackwell; 2011. pp. 43–59.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

