Redirecting TGF- β Signaling through the β-Catenin/Foxo Complex Prevents Kidney Fibrosis
- PMID: 29180394
- PMCID: PMC5791062
- DOI: 10.1681/ASN.2016121362
Redirecting TGF- β Signaling through the β-Catenin/Foxo Complex Prevents Kidney Fibrosis
Abstract
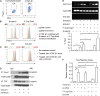
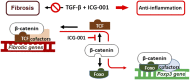
TGF-β is a key profibrotic factor, but targeting TGF-β to prevent fibrosis also abolishes its protective anti-inflammatory effects. Here, we investigated the hypothesis that we can redirect TGF-β signaling by preventing downstream profibrotic interaction of β-catenin with T cell factor (TCF), thereby enhancing the interaction of β-catenin with Foxo, a transcription factor that controls differentiation of TGF-β induced regulatory T cells (iTregs), and thus, enhance anti-inflammatory effects of TGF-β In iTregs derived from EL4 T cells treated with recombinant human TGF-β1 (rhTGF-β1) in vitro, inhibition of β-catenin/TCF transcription with ICG-001 increased Foxp3 expression, interaction of β-catenin and Foxo1, binding of Foxo1 to the Foxp3 promoter, and Foxo transcriptional activity. Moreover, the level of β-catenin expression positively correlated with the level of Foxo1 binding to the Foxp3 promoter and Foxo transcriptional activity. T cell fate mapping in Foxp3gfp Ly5.1/5.2 mice revealed that coadministration of rhTGF-β1 and ICG-001 further enhanced the expansion of iTregs and natural Tregs observed with rhTGF-β1 treatment alone. Coadministration of rhTGF-β1 with ICG-001 also increased the number of Tregs and reduced inflammation and fibrosis in the kidney fibrosis models of unilateral ureteric obstruction and ischemia-reperfusion injury. Notably, ICG-001 prevented the fibrosis in distant organs (lung and liver) caused by rhTGF-β1. Together, our results show that diversion of β-catenin from TCF- to Foxo-mediated transcription inhibits the β-catenin/TCF-mediated profibrotic effects of TGF-β while enhancing the β-catenin/Foxo-mediated anti-inflammatory effects. Targeting β-catenin/Foxo may be a novel therapeutic strategy in the treatment of fibrotic diseases that lead to organ failure.
Keywords: TGF-β; chronic kidney disease; fibrosis.
Copyright © 2018 by the American Society of Nephrology.
Figures







References
-
- Yanagita M: Inhibitors/antagonists of TGF-β system in kidney fibrosis. Nephrol Dial Transplant 27: 3686–3691, 2012 - PubMed
-
- Border WA, Noble NA: Transforming growth factor beta in tissue fibrosis. N Engl J Med 331: 1286–1292, 1994 - PubMed
-
- Meng XM, Nikolic-Paterson DJ, Lan HY: TGF-β: The master regulator of fibrosis. Nat Rev Nephrol 12: 325–338, 2016 - PubMed
-
- Vincenti F, Fervenza FC, Campbell KN, Diaz M, Gesualdo L, Nelson P, Praga M, Radhakrishnan J, Sellin L, Singh A, Thornley-Brown D, Veronese FV, Accomando B, Engstrand S, Ledbetter S, Lin J, Neylan J, Tumlin J; Focal Segmental Glomerulosclerosis Study Group : A phase 2, double-blind, placebo-controlled, randomized study of fresolimumab in patients with steroid-resistant primary focal segmental glomerulosclerosis. Kidney Int Rep 2: 800–810, 2017 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

