Tamm-Horsfall Protein Regulates Mononuclear Phagocytes in the Kidney
- PMID: 29180395
- PMCID: PMC5827593
- DOI: 10.1681/ASN.2017040409
Tamm-Horsfall Protein Regulates Mononuclear Phagocytes in the Kidney
Abstract
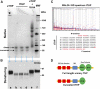
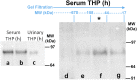
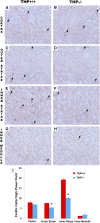
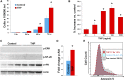
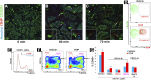
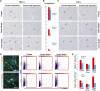
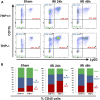
Tamm-Horsfall protein (THP), also known as uromodulin, is a kidney-specific protein produced by cells of the thick ascending limb of the loop of Henle. Although predominantly secreted apically into the urine, where it becomes highly polymerized, THP is also released basolaterally, toward the interstitium and circulation, to inhibit tubular inflammatory signaling. Whether, through this latter route, THP can also regulate the function of renal interstitial mononuclear phagocytes (MPCs) remains unclear, however. Here, we show that THP is primarily in a monomeric form in human serum. Compared with wild-type mice, THP-/- mice had markedly fewer MPCs in the kidney. A nonpolymerizing, truncated form of THP stimulated the proliferation of human macrophage cells in culture and partially restored the number of kidney MPCs when administered to THP-/- mice. Furthermore, resident renal MPCs had impaired phagocytic activity in the absence of THP. After ischemia-reperfusion injury, THP-/- mice, compared with wild-type mice, exhibited aggravated injury and an impaired transition of renal macrophages toward an M2 healing phenotype. However, treatment of THP-/- mice with truncated THP after ischemia-reperfusion injury mitigated the worsening of AKI. Taken together, our data suggest that interstitial THP positively regulates mononuclear phagocyte number, plasticity, and phagocytic activity. In addition to the effect of THP on the epithelium and granulopoiesis, this new immunomodulatory role could explain the protection conferred by THP during AKI.
Keywords: acute renal failure; ischemia-reperfusion; macrophages.
Copyright © 2018 by the American Society of Nephrology.
Figures





References
-
- Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O: The rediscovery of uromodulin (Tamm-Horsfall protein): From tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 80: 338–347, 2011 - PubMed
-
- Zhu X, Cheng J, Gao J, Lepor H, Zhang ZT, Pak J, Wu XR: Isolation of mouse THP gene promoter and demonstration of its kidney-specific activity in transgenic mice. Am J Physiol Renal Physiol 282: F608–F617, 2002 - PubMed
-
- Bachmann S, Koeppen-Hagemann I, Kriz W: Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry 83: 531–538, 1985 - PubMed
-
- El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR: Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol 304: F1066–F1075, 2013 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

