Inhibition of heme oxygenase ameliorates anemia and reduces iron overload in a β-thalassemia mouse model
- PMID: 29180398
- PMCID: PMC5757685
- DOI: 10.1182/blood-2017-07-798728
Inhibition of heme oxygenase ameliorates anemia and reduces iron overload in a β-thalassemia mouse model
Abstract
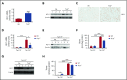
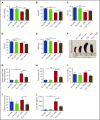
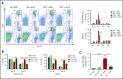
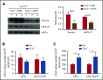
Thalassemias are a heterogeneous group of red blood cell disorders, considered a major cause of morbidity and mortality among genetic diseases. However, there is still no universally available cure for thalassemias. The underlying basis of thalassemia pathology is the premature apoptotic destruction of erythroblasts causing ineffective erythropoiesis. In β-thalassemia, β-globin synthesis is reduced causing α-globin accumulation. Unpaired globin chains, with heme attached to them, accumulate in thalassemic erythroblasts causing oxidative stress and the premature cell death. We hypothesize that in β-thalassemia heme oxygenase (HO) 1 could play a pathogenic role in the development of anemia and ineffective erythropoiesis. To test this hypothesis, we exploited a mouse model of β-thalassemia intermedia, Th3/+ We observed that HO inhibition using tin protoporphyrin IX (SnPP) decreased heme-iron recycling in the liver and ameliorated anemia in the Th3/+ mice. SnPP administration led to a decrease in erythropoietin and increase in hepcidin serum levels, changes that were accompanied by an alleviation of ineffective erythropoiesis in Th3/+ mice. Additionally, the bone marrow from Th3/+ mice treated with SnPP exhibited decreased heme catabolism and diminished iron release as well as reduced apoptosis. Our results indicate that the iron released from heme because of HO activity contributes to the pathophysiology of thalassemia. Therefore, new therapies that suppress heme catabolism may be beneficial in ameliorating the anemia and ineffective erythropoiesis in thalassemias.
© 2018 by The American Society of Hematology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

