A neutralizing antibody that blocks delivery of the enzymatic cargo of Clostridium difficile toxin TcdB into host cells
- PMID: 29180448
- PMCID: PMC5777265
- DOI: 10.1074/jbc.M117.813428
A neutralizing antibody that blocks delivery of the enzymatic cargo of Clostridium difficile toxin TcdB into host cells
Abstract
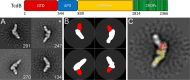
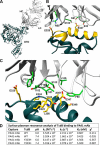
Clostridium difficile infection is the leading cause of hospital-acquired diarrhea and is mediated by the actions of two toxins, TcdA and TcdB. The toxins perturb host cell function through a multistep process of receptor binding, endocytosis, low pH-induced pore formation, and the translocation and delivery of an N-terminal glucosyltransferase domain that inactivates host GTPases. Infection studies with isogenic strains having defined toxin deletions have established TcdB as an important target for therapeutic development. Monoclonal antibodies that neutralize TcdB function have been shown to protect against C. difficile infection in animal models and reduce recurrence in humans. Here, we report the mechanism of TcdB neutralization by PA41, a humanized monoclonal antibody capable of neutralizing TcdB from a diverse array of C. difficile strains. Through a combination of structural, biochemical, and cell functional studies, involving X-ray crystallography and EM, we show that PA41 recognizes a single, highly conserved epitope on the TcdB glucosyltransferase domain and blocks productive translocation and delivery of the enzymatic cargo into the host cell. Our study reveals a unique mechanism of C. difficile toxin neutralization by a monoclonal antibody, which involves targeting a process that is conserved across the large clostridial glucosylating toxins. The PA41 antibody described here provides a valuable tool for dissecting the mechanism of toxin pore formation and translocation across the endosomal membrane.
Keywords: X-ray crystallography; antibody; bacterial pathogenesis; bacterial toxin; electron microscopy (EM); membrane transport; neutralization; toxin.
Conflict of interest statement
These studies were partially funded by MedImmune through a collaborative project awarded to D. B. L. MedImmune had the right to review the manuscript but otherwise had no role in the preparation or decision to publish. K. R., R. W., X. J., A. C. N., G. J. R., and P. W. are employees of MedImmune, a unit of AstraZeneca. K. R., R. W., X. J., A. C. N., G. J. R., and P. W. own stock in AstraZeneca. G. J. R. is now employed by MabVax Therapeutics
Figures




References
-
- Lessa F. C., Mu Y., Bamberg W. M., Beldavs Z. G., Dumyati G. K., Dunn J. R., Farley M. M., Holzbauer S. M., Meek J. I., Phipps E. C., Wilson L. E., Winston L. G., Cohen J. A., Limbago B. M., Fridkin S. K., et al. (2015) Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 372, 825–834 10.1056/NEJMoa1408913 - DOI - PMC - PubMed
-
- Carter G. P., Chakravorty A., Pham Nguyen T. A., Mileto S., Schreiber F., Li L., Howarth P., Clare S., Cunningham B., Sambol S. P., Cheknis A., Figueroa I., Johnson S., Gerding D., Rood J. I., et al. (2015) Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. MBio 6, e00551.10.1128/mBio.00551-15 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

