Migration against the direction of flow is LFA-1-dependent in human hematopoietic stem and progenitor cells
- PMID: 29180515
- PMCID: PMC5818057
- DOI: 10.1242/jcs.205575
Migration against the direction of flow is LFA-1-dependent in human hematopoietic stem and progenitor cells
Erratum in
-
Correction: Migration against the direction of flow is LFA-1-dependent in human hematopoietic stem and progenitor cells (doi: 10.1242/jcs.205575).J Cell Sci. 2018 Sep 10;131(17):jcs224204. doi: 10.1242/jcs.224204. J Cell Sci. 2018. PMID: 30201798 Free PMC article. No abstract available.
Abstract
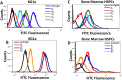
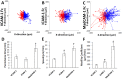
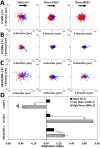
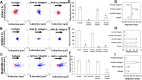
The recruitment of immune cells during inflammation is regulated by a multi-step cascade of cell rolling, activation, adhesion and transmigration through the endothelial barrier. Similarly, hematopoietic stem and progenitor cells (HSPCs) use this pathway to migrate and home to the bone marrow. After selectin-mediated braking, HSPCs migrate on adhesion ligands presented by the vascular endothelium including ICAM-1, VCAM-1 or MAdCAM-1. Here, we report that both the KG1a stem cell line and primary bone marrow CD34+ HSPCs can migrate against the direction of fluid flow on surfaces coated with cell adhesion molecules (CAMs), a behavior thus far only reported in T lymphocytes. We demonstrate that KG1a cells and primary HSPCs migrate upstream on surfaces presenting ICAM-1, downstream on surfaces presenting VCAM-1, and both upstream and downstream on surfaces presenting MAdCAM-1. In addition, we demonstrate that KG1a cells and HSPCs display upstream migration both on surfaces with multiple CAMs, as well as on human umbilical vein endothelial cell (HUVEC) monolayers. By blocking with monoclonal antibodies, we show that lymphocyte function-associated antigen-1 (LFA-1) is the key receptor responsible for upstream migration on the endothelium during the trafficking of HSPCs to the bone marrow.This article has an associated First Person interview with the first author of the paper.
Keywords: Cell migration; Endothelium; Hematopoietic stem and progenitor cell; Homing; ICAM-1; Inflammation; LFA-1; MAdCAM-1; VCAM-1.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




Similar articles
-
Integrin crosstalk allows CD4+ T lymphocytes to continue migrating in the upstream direction after flow.Integr Biol (Camb). 2019 Dec 31;11(10):384-393. doi: 10.1093/intbio/zyz034. Integr Biol (Camb). 2019. PMID: 31851360 Free PMC article.
-
Human Neutrophils Will Crawl Upstream on ICAM-1 If Mac-1 Is Blocked.Biophys J. 2019 Oct 15;117(8):1393-1404. doi: 10.1016/j.bpj.2019.08.044. Epub 2019 Sep 18. Biophys J. 2019. PMID: 31585707 Free PMC article.
-
The direction of migration of T-lymphocytes under flow depends upon which adhesion receptors are engaged.Integr Biol (Camb). 2015 Mar;7(3):345-55. doi: 10.1039/c4ib00201f. Epub 2015 Feb 12. Integr Biol (Camb). 2015. PMID: 25674729 Free PMC article.
-
Atherosclerosis and inflammation mononuclear cell recruitment and adhesion molecules with reference to the implication of ICAM-1/LFA-1 pathway in atherogenesis.Int J Cardiol. 1998 Oct 1;66 Suppl 1:S45-53; discussion S55. doi: 10.1016/s0167-5273(98)00147-8. Int J Cardiol. 1998. PMID: 9951802 Review.
-
In vitro models of lymphocyte transendothelial migration.Invasion Metastasis. 1992;12(2):66-81. Invasion Metastasis. 1992. PMID: 1383172 Review.
Cited by
-
Integrin crosstalk allows CD4+ T lymphocytes to continue migrating in the upstream direction after flow.Integr Biol (Camb). 2019 Dec 31;11(10):384-393. doi: 10.1093/intbio/zyz034. Integr Biol (Camb). 2019. PMID: 31851360 Free PMC article.
-
Human Gut-Associated Natural Killer Cells in Health and Disease.Front Immunol. 2019 May 3;10:961. doi: 10.3389/fimmu.2019.00961. eCollection 2019. Front Immunol. 2019. PMID: 31130953 Free PMC article. Review.
-
Functional Mapping of Adhesiveness on Live Cells Reveals How Guidance Phenotypes Can Emerge From Complex Spatiotemporal Integrin Regulation.Front Bioeng Biotechnol. 2021 Apr 7;9:625366. doi: 10.3389/fbioe.2021.625366. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33898401 Free PMC article.
-
Human Neutrophils Will Crawl Upstream on ICAM-1 If Mac-1 Is Blocked.Biophys J. 2019 Oct 15;117(8):1393-1404. doi: 10.1016/j.bpj.2019.08.044. Epub 2019 Sep 18. Biophys J. 2019. PMID: 31585707 Free PMC article.
-
T lymphocytes migrate upstream after completing the leukocyte adhesion cascade.Cell Adh Migr. 2019 Dec;13(1):163-168. doi: 10.1080/19336918.2019.1587269. Epub 2019 Mar 17. Cell Adh Migr. 2019. PMID: 30879410 Free PMC article.
References
-
- Briskin M. J., Rott L. and Butcher E. C. (1996). Structural requirements for mucosal vascular addressin binding to its lymphocyte receptor α4β7: common themes among integrin-Ig family interactions. J. Immunol. 156, 719-726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

