Safety and Pharmacokinetics of the Aminomethylcycline Antibiotic Omadacycline Administered to Healthy Subjects in Oral Multiple-Dose Regimens
- PMID: 29180524
- PMCID: PMC5786815
- DOI: 10.1128/AAC.01487-17
Safety and Pharmacokinetics of the Aminomethylcycline Antibiotic Omadacycline Administered to Healthy Subjects in Oral Multiple-Dose Regimens
Abstract
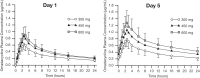
Omadacycline, a first-in-class aminomethylcycline antibiotic, is related to tetracyclines but is structurally modified to circumvent mechanisms of resistance to tetracyclines. Omadacycline demonstrates potent activity against a broad range of pathogens, including drug-resistant strains, and is in late-stage development for treatment of acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia. Previous studies support an intravenous-to-oral transition regimen with 300-mg once-daily oral dosing. This phase 1 study investigated the pharmacokinetics and safety/tolerability of multiple oral omadacycline doses higher than 300 mg. Using a 3-period crossover design, healthy adults were randomized to receive oral omadacycline at 300, 450, and 600 mg in variable sequence (n = 26) or placebo (n = 7) once daily for 5 consecutive days per period. In plasma, omadacycline maximum concentration and total exposure increased with increasing dose but were less than dose proportional. The kinetics of omadacycline plasma accumulation were similar between dose levels; exposure on day 5 was ∼50% higher than that on day 1. Omadacycline plasma concentrations on day 1 of 450-mg dosing were similar to those on day 5 of 300-mg dosing. All doses were generally well tolerated, but the 600-mg dose was associated with more gastrointestinal adverse events.
Keywords: multidose regimen; omadacycline; oral dosing.
Copyright © 2018 Bundrant et al.
Figures
Similar articles
-
Omadacycline: A Review of the Clinical Pharmacokinetics and Pharmacodynamics.Clin Pharmacokinet. 2020 Apr;59(4):409-425. doi: 10.1007/s40262-019-00843-4. Clin Pharmacokinet. 2020. PMID: 31773505 Review.
-
Randomized, Open-Label Study of the Pharmacokinetics and Safety of Oral and Intravenous Administration of Omadacycline to Healthy Subjects.Antimicrob Agents Chemother. 2016 Nov 21;60(12):7431-7435. doi: 10.1128/AAC.01393-16. Print 2016 Dec. Antimicrob Agents Chemother. 2016. PMID: 27736760 Free PMC article. Clinical Trial.
-
Pharmacokinetics and Safety of Omadacycline in Subjects with Impaired Renal Function.Antimicrob Agents Chemother. 2018 Jan 25;62(2):e02057-17. doi: 10.1128/AAC.02057-17. Print 2018 Feb. Antimicrob Agents Chemother. 2018. PMID: 29158281 Free PMC article.
-
Omadacycline: A Novel Oral and Intravenous Aminomethylcycline Antibiotic Agent.Drugs. 2020 Feb;80(3):285-313. doi: 10.1007/s40265-020-01257-4. Drugs. 2020. PMID: 31970713 Review.
-
Pharmacokinetics, Distribution, Metabolism, and Excretion of Omadacycline following a Single Intravenous or Oral Dose of 14C-Omadacycline in Rats.Antimicrob Agents Chemother. 2016 Dec 27;61(1):e01784-16. doi: 10.1128/AAC.01784-16. Print 2017 Jan. Antimicrob Agents Chemother. 2016. PMID: 27821446 Free PMC article.
Cited by
-
Pharmacokinetics, Safety and Pharmacokinetics/Pharmacodynamics Analysis of Omadacycline in Chinese Healthy Subjects.Front Pharmacol. 2022 Apr 21;13:869237. doi: 10.3389/fphar.2022.869237. eCollection 2022. Front Pharmacol. 2022. PMID: 35529438 Free PMC article.
-
Omadacycline Oral Dosing and Pharmacokinetics in Community-Acquired Bacterial Pneumonia and Acute Bacterial Skin and Skin Structure Infection.Clin Drug Investig. 2022 Mar;42(3):193-197. doi: 10.1007/s40261-022-01119-9. Epub 2022 Feb 22. Clin Drug Investig. 2022. PMID: 35192150 Free PMC article. Review.
-
Pharmacokinetics and Pharmacodynamics of Oral and Intravenous Omadacycline.Clin Infect Dis. 2019 Aug 1;69(Suppl 1):S16-S22. doi: 10.1093/cid/ciz309. Clin Infect Dis. 2019. PMID: 31367744 Free PMC article. Review.
-
Efficacy and safety of omadacycline for treating complicated skin and soft tissue infections: a meta-analysis of randomized controlled trials.BMC Infect Dis. 2024 Feb 19;24(1):219. doi: 10.1186/s12879-024-09097-3. BMC Infect Dis. 2024. PMID: 38374030 Free PMC article.
-
Omadacycline: A Review of the Clinical Pharmacokinetics and Pharmacodynamics.Clin Pharmacokinet. 2020 Apr;59(4):409-425. doi: 10.1007/s40262-019-00843-4. Clin Pharmacokinet. 2020. PMID: 31773505 Review.
References
-
- Honeyman L, Ismail M, Nelson ML, Bhatia B, Bowser TE, Chen J, Mechiche R, Ohemeng K, Verma AK, Cannon EP, Macone A, Tanaka SK, Levy S. 2015. Structure-activity relationship of the aminomethylcyclines and the discovery of omadacycline. Antimicrob Agents Chemother 59:7044–7053. doi:10.1128/AAC.01536-15. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

