Intracellular Distribution of Manganese by the Trans-Golgi Network Transporter NRAMP2 Is Critical for Photosynthesis and Cellular Redox Homeostasis
- PMID: 29180598
- PMCID: PMC5757278
- DOI: 10.1105/tpc.17.00578
Intracellular Distribution of Manganese by the Trans-Golgi Network Transporter NRAMP2 Is Critical for Photosynthesis and Cellular Redox Homeostasis
Abstract
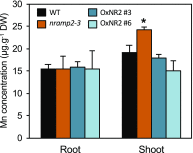
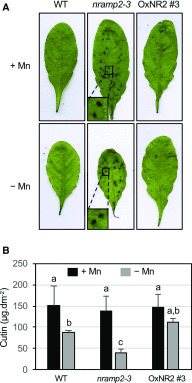
Plants require trace levels of manganese (Mn) for survival, as it is an essential cofactor in oxygen metabolism, especially O2 production via photosynthesis and the disposal of superoxide radicals. These processes occur in specialized organelles, requiring membrane-bound intracellular transporters to partition Mn between cell compartments. We identified an Arabidopsis thaliana member of the NRAMP family of divalent metal transporters, NRAMP2, which functions in the intracellular distribution of Mn. Two knockdown alleles of NRAMP2 showed decreased activity of photosystem II and increased oxidative stress under Mn-deficient conditions, yet total Mn content remained unchanged. At the subcellular level, these phenotypes were associated with a loss of Mn content in vacuoles and chloroplasts. NRAMP2 was able to rescue the mitochondrial yeast mutant mtm1∆ In plants, NRAMP2 is a resident protein of the trans-Golgi network. NRAMP2 may act indirectly on downstream organelles by building up a cytosolic pool that is used to feed target compartments. Moreover, not only does the nramp2 mutant accumulate superoxide ions, but NRAMP2 can functionally replace cytosolic superoxide dismutase in yeast, indicating that the pool of Mn displaced by NRAMP2 is required for the detoxification of reactive oxygen species.
© 2017 American Society of Plant Biologists. All rights reserved.
Figures

Comment in
-
Manganese Is a Plant's Best Friend: Intracellular Mn Transport by the Transporter NRAMP2.Plant Cell. 2017 Dec;29(12):2953-2954. doi: 10.1105/tpc.17.00965. Epub 2017 Dec 14. Plant Cell. 2017. PMID: 29242315 Free PMC article. No abstract available.
References
-
- Agorio A., Giraudat J., Bianchi M.W., Marion J., Espagne C., Castaings L., Lelièvre F., Curie C., Thomine S., Merlot S. (2017). Phosphatidylinositol 3-phosphate-binding protein AtPH1 controls the localization of the metal transporter NRAMP1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 114: E3354–E3363. - PMC - PubMed
-
- Barberon M., Vermeer J.E., De Bellis D., Wang P., Naseer S., Andersen T.G., Humbel B.M., Nawrath C., Takano J., Salt D.E., Geldner N. (2016). Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164: 447–459. - PubMed
-
- Bolte S., Cordelières F.P. (2006). A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224: 213–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

